Abstract
Acute gastroenteritis is an infectious disease of the alimentary tract that may be caused by one or more bacterial, viral, or protozoal pathogens producing damage, either structural or functional, of variable extent and severity to the mucosa. Acute gastroenteritis is a very common diagnosis in pediatric practices. It is usually a clinical diagnosis. Its most common symptoms are acute vomiting and diarrhea. The most important acute complication of gastroenteritis is dehydration, which occurs when the child's overall output of fluid exceeds input. Insensible water loss is the volume of fluid that leaves the body as a result of the difference in vapor pressure between the skin and lung surfaces and the surrounding atmosphere. The small intestine is the organ principally affected in most children by gastroenteritis but the stomach and colon may also be involved to a varying extent. Most often acute gastroenteritis is a self-limiting illness and may result in full recovery after 24 hours.
Acute gastroenteritis is a very common diagnosis in paediatric practices. It is usually a clinical diagnosis. Its commonest symptoms are acute vomiting and diarrhoea, which are such frequent symptoms in infancy that children having them may incorrectly be labelled as suffering from acute gastroenteritis without any real justification. It is thus important to define what is meant by acute gastroenteritis, although it must be appreciated that clinically it may be difficult at times to be certain of the diagnosis.
Definition
Acute gastroenteritis is an infectious disease of the alimentary tract which may be caused by one or more bacterial, viral or protozoal pathogens producing damage to the mucosa either structural or functional of variable extent and severity.
Another definition of acute gastroenteritis is the clinical syndrome of diarrhoea and/or vomiting of acute onset, often accompanied by fever and constitutional disturbance which is of infective origin and is not secondary to some primary disease process outside the alimentary tract.
Aetiology
Role of bacteria
No bacterial pathogen can be isolated from the stools of the majority of children with gastroenteritis who are admitted to hospital in a developed country, but in developing countries over 50% of children may have bacterial isolations.
Table 6.1 indicates the percentage bacterial isolation from the stools of children with gastroenteritis admitted to children's hospitals in Britain, Australia, Indonesia and Bangladesh.
Table 6.1.
Bacterial isolation in gastroenteritis
| Australia (Sydney) | Britain (London) | Indonesia (Bandung) | Bangladesh (Dacca) | |
|---|---|---|---|---|
| Author | Dorman (1968) | Walker-Smith (unpublished observations) | Suprapti et al. (1968) | Stoll et al. (1982) |
| No. of children | 828 | 530 | 466 | 2624 |
| Percentage isolation: | ||||
| Salmonella | 12 | 2.2 | 2.4 | 1 |
| Shigella | 6 | 3.4 | 11.2 | 11 |
| E. coli | ||||
| Enteropathogenic | 7.5 | 6.4 | 32.2 | ND |
| Enterotoxigenic | ND | ND | ND | 20 |
| Campylobacter | ND | ND | ND | 17 |
| Cholera | — | — | 0.9 | 7 |
ND: Not done.
The percentage isolation of enteropathogenic Escherichia coli is far higher in the Indonesian series than the British or Australian reports, while the percentage isolation of salmonella is higher in the Australian series than in the other reports. This may reflect the popularity of frozen poultry in Australia. Some authorities no longer report the finding of the classical strains of enteropathogenic E. coli, for example this was not done in the Bangladeshi report. Others have not studied enterotoxigenic E. coli. Thus a total comprehensive survey comparing the aetiology in developing and developed communities is lacking. The high prevalence of bacterial pathogens in the Indonesian study related to the remarkably high level of isolation of enteropathogenic E. coli. In any event the high percentage of bacterial isolation in the Indonesian series is related to the prevalence of malnutrition and poor hygiene in the community. This is well illustrated by the observations of Gracey (1973) in young malnourished aboriginals with acute diarrhoea in Australia, where there is a similar prevalence of malnutrition and poor hygiene. He found that from a group of 251 such patients, 47 excreted a serotype of enteropathogenic E. coli, 17 a species of salmonella and 25 a shigella. A similar high isolation rate of bacterial pathogens in another developing community was reported by Maiya et al. (1976), who found recognized bacterial pathogens in the stools of 66% of a group of children under 2 years of age with acute gastroenteritis in Southern India, observed overa 12-month period.
Infection with individual bacterial pathogens will be discussed in more detail later, but the mere isolation of a known bacterial pathogen from the stool of a child with acute gastroenteritis does not establish that it is the causative agent of the syndrome. Feldman, Bhat and Kamath (1970) in South India have shown a dissociation between the pattern of clinical illness and the pattern of isolation of bacterial pathogens in an impressive epidemiological survey of pre-school Indian children. However, such isolation does provide good presumptive evidence of a cause and effect relationship at the time of an outbreak of infective diarrhoea when other children are found to excrete the same pathogen, but care has to be taken when interpreting the significance of the results of stool culture taken from an individual child with acute diarrhoea and vomiting.
There are important differences in the manner in which these bacterial pathogens may produce their toxic effects. Salmonellae penetrate the mucosa relatively deeply but do not produce an enterotoxin whereas Shigella shiga produces a powerful enterotoxin which may cause ileal hypersecretion, almost to the same degree as that which occurs in cholera. It is believed that it is this penetration or invasion of the mucosa by salmonellae, and some strains of enteropathogenic E. coli and most shigellae, that permits the endotoxin they produce to exert its toxic effect.
Thus salmonellae, shigellae and some strains of E. coli may invade the intestinal epithelial cell and multiply within the mucosa. Animal studies have greatly helped our understanding of the pathogenetic mechanisms involved. Formal et al. (1976) have shown that in both salmonellosis and shigellosis bacterial invasion of the colonic mucosa does occur. This is associated with acute inflammatory reaction and mucosal damage and, in turn, there is abnormal colonic salt and water transport. In the animals studied they have also found that the jejunum is in a net secretory state despite the absence of bacterial invasion or morphological abnormalities in the jejunum. In these circumstances the diarrhoea produced by invasive organisms results from the inability of the colon to reabsorb the increased volume of fluid entering the colon from the small intestine.
Enterotoxins are synthesized within the bacterial cell body and are elaborated into broth cultures containing intact bacteria, whereas endotoxins are associated within the bacterial cell wall and so are not found in broths unless there is damage or destruction of the bacteria. Enterotoxins have classically been shown to be produced by Vibrio cholerae but also by some strains of E. coli and food poisoning strains of Staphylococcus aureus and Clostridium perfringens as well as Shigella shiga.
One of the most fascinating observations in this field in recent years has been the demonstration that cholera enterotoxin produces its hypersecretory effect via 3′-5′ adenosine monophosphate (cyclic AMP). Field (1971) and others have shown that cholera enterotoxin increases intestinal levels of cyclic AMP. It also has been shown to activate adenyl cyclase, the enzyme that converts adenosine triphosphate (ATP) into cyclic AMP. It is this activation of adenyl cyclase which accounts for the fluid hypersecretion by the gut.
Invasive enteropathogens such as salmonellae as well as enterotoxin-producing bacteria like Vibrio cholerae can produce elevated levels of adenylate cyclase activity. This could result from stimulation by prostaglandins synthesized locally. Indomethacin, a potent inhibitor of prostaglandin synthesis, has been shown in the experimental animal to abolish adenylate cyclase activation and fluid secretion induced by Salmonella typhimurium (Gianella et al., 1975). Duguid and Gillies for the first time in 1957 showed that Shigella dysenteriae, enteropathogenic E. coli and Enterobacter species could adhere to human intestinal mucosal cells and also that surface filaments called fimbriae or pili were the adhesive structure.
The attachment is via surface proteins or adhesins. The ability of bacteria to adhere to the intestinal mucosa is an essential prerequisite for colonization of the small intestine and for pathogenicity. McNeish et al. in 1975 showed for the first time the relevance of bacterial adhesion to human disease. They showed that diarrhoeagenic E. coli could adhere to human foetal intestine whereas non-entero-pathogenic strains of E. coli did not. Species specificity was shown inasmuch that E. coli which adhered to human small intestine did not adhere to brush border preparations from the small intestine of guinea-pigs, rabbits, calves and pigs. The fact that adhesion of enteropathogenic E. coli was mediated by a 60-megadalton plasmid was later established (Williams et al., 1978). A plasmid is an extra-chromosomal genetic element. Knutton et al. (1984) have developed a practical in vitro assay for adhesion. Adhesion is clearly an important early event for colonization by bacteria of the intestine.
The importance of this is eloquently shown by the example of enterotoxigenic E. coli which cause life-threatening diarrhoea when they are adhesive but are completely non-pathogenic when they are not adhesive. A host factor may also be important as has best been shown in piglets where certain litters of piglets had brush border membranes to which K88 enterotoxigenic E. coli were unable to adhere to (Sellwood et al., 1975).
So, to summarize, two mechanisms have been recognized whereby bacterial pathogens may produce the syndromes of acute gastroenteritis. These are toxin production and mucosal invasion. The particular organisms may thus be designated toxigenic or invasive. However a vital factor in pathogenicity is preliminary bacterial adhesion.
Sometimes bacteria not ordinarily regarded as pathogens may be associated with outbreaks of diarrhoea. These include Pseudomonas aeruginosa, especially in premature infants, and some strains of klebsiella. This could relate to transfer of plasmids from known pathogens to these organisms.
Bacterial pathogens, not previously known to be aetiological agents for acute gastroenteritis were identified in the 1970s. These are Yersinia enter ocolitica, Campylobacter (Vantrappen et al., 1977; Tauwers, De Boeck and Butzler, 1978) and Aeromonas (Gracey et al., 1982).
Role of viruses
As mentioned earlier it has been known for some years now in developed countries that in only a minority of children with acute gastroenteritis can bacterial pathogens be isolated from the stools. The role of viruses in the remaining children with nonbacterial gastroenteritis was uncertain until the early 1970s.
Exciting new work was reported from the United States by Blacklow and his colleagues in 1972 who studied an outbreak of winter vomiting disease among school children in Norwalk, Ohio. They found that bacteria-free stool filtrates derived from people infected during this outbreak led to acute vomiting and diarrhoea when administered to human volunteers. The infectious agent in this outbreak was then established by means of immune electron microscopy to be a virus which has come to be known as the Norwalk agent or virus (Kapikian et al., 1972).
Bishop and her colleagues (1973) from Melbourne using the electron microscope found virus particles in the epithelial cells of duodenal mucosa obtained on small intestinal biopsy from 6 out of 9 children with gastroenteritis (Figure. 6.16 ). The virus particles were found more readily in small intestinal biopsies obtained early after the onset of symptoms in these infants and less readily in those who had a longer history at the time of biopsy.
Figure 6.16.
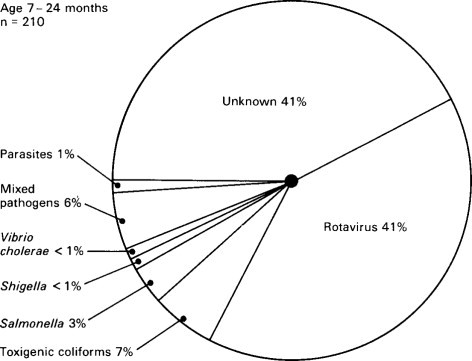
Relative importance of infectious agents in children aged 7–24 months with diarrhoea requiring admission to hospital in Indonesia.
(Reproduced from Soenarto et al. (1983) by kind permission of authors and publishers.)
© 1988
Bishop and her colleagues in 1974 went on to use electron microscopy of negatively stained faecal extracts to reveal these particles in 11 out of 14 children aged less than 3 years who had acute non-bacterial gastroenteritis. Independently, in Birmingham, Flewett, Bryden and Davies (1973) found these particles and called them rotavirus from the Latin rota, a wheel (Figure. 6.1 ). Reports of similar particles in the stools of children came rapidly from around the world. It is now clear that rotavirus infection is the most common cause of acute diarrhoea in developed communities. Whilst in developing communities it is only enterotoxigenic E. coli infections which can rival rotavirus as significant identifiable aetiological agents for acute gastroenteritis.
Figure 6.1.
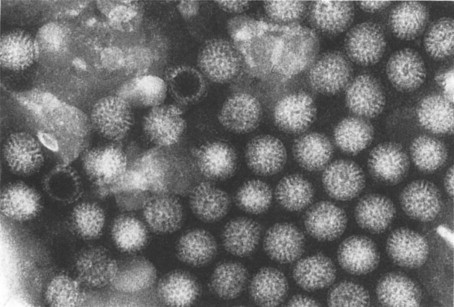
Rotavirus particles in stool demonstrated by electron microscopy, × 240 000.
(Reproduced by kind permission of Phillips.)
Population based prospective studies in North America have shown annual rates of diarrhoea for the first year of life to be 0.82-0.86 per child for an area of southern Michigan (Koopman et al., 1984) and 1.05 per child for a region of Winnipeg (Gurwith et al., 1981). Rotavirus was identified in 19% and 31% respectively of these studies.
The technique for examining negatively stained faecal extracts with the electron microscope for the presence of virus particles has now been widely applied in the investigation of diarrhoeal states in children with gastroenteritis, and a number of viruses have been recognized by their morphological appearance (Madeley et al., 1975).
At Queen Elizabeth Hospital for Children, at present as well as rotavirus, adenovirus, astrovirus, Norwalk-like agents, calici virus, small round unstructured viruses and coronavirus are all recognized by their characteristic features on electron microscopy (Table 6.2 ). While discussing the possible viral aetiology of gastroenteritis, it is important to recall that acute diarrhoea may accompany a number of viral illnesses. In developing communities, where measles is often a severe illness and a common problem, acute diarrhoea is a frequent manifestation of the malady (Axton, 1986). In developed communities, diarrhoea is usually a less important manifestation of measles but there are occasional children in whom severe diarrhoea accompanies measles.
Table 6.2.
Stool virus identification by electron microscopy (March 1982 to September 1983) at Queen Elizabeth Hospital for Children, London
| No. | % | |
|---|---|---|
| Rotavirus | 268 | (53.4%) |
| Adenovirus | 80 | (15.9%) |
| Astrovirus | 61 | (12.2%) |
| Norwalk agent | 42 | (8.4%) |
| Calicivirus | 19 | (3.8%) |
| Small round unstructured virus | 17 | (3.4%) |
| Coronavirus | 15 | (3.0%) |
Giles, Monif and Hood (1970) reported post-mortem findings in a child who developed severe diarrhoea with measles, followed by sudden death. The ilecolitis diagnosed on histopathological section was attributed to the measles virus.
In adults, Sheeky, Artenstein and Green (1964) have studied small intestinal mucosal morphology using biopsy in a variety of viral illnesses and they have found a significant incidence of abnormal findings in a number of viral illness including infectious hepatitis and measles.
The current pattern for identification of stool pathogens both bacterial and viral for in-patients at Queen Elizabeth Hospital is indicated in Table 6.3 .
Table 6.3.
Stool pathogens in gastroenteritis (Queen Elizabeth Hospital)
| No. | % | |
|---|---|---|
| Total studied | 200 | |
| Bacteria: | ||
| Salmonella | 16 | 8 |
| Shigella | 16 | 8 |
| Enteropathogenic E.coli | 19 | 9.5 |
| Campylobacter | 11 | 5.5 |
| Other | 1 | 0.5 |
| No growth | 73 | |
| Not done | 9 | 4.5 |
| Viruses: | ||
| Rota | 45 | 22.5 |
| Adeno | 16 | 8 |
| Astro | 3 | 1.5 |
| Calici | 5 | 2.5 |
| Small round | 4 | 2 |
| Not done | 36 | 18 |
| Negative | 94 | 47 |
| Total pathogens in 166 children who had virology and bacteriology = 99 children, i.e. 59.6% | ||
Source: Trounce and Walker-Smith (1985).
© 1988
Pathology
The small intestine is the organ principally affected in most children by gastroenteritis but the stomach and colon may also be involved to a varying extent. An exception may be shigellosis where the brunt of the disease is borne by the colon. Barnes (1973), in a study of 21 children with non-bacterial gastroenteritis, found evidence of inflammation in stomach, duodenum and rectum of some children, indicating that the disease may affect the whole gastrointestinal tract. In particular, in 15 of 21 biopsies there was some inflammation of the stomach.
This variable distribution of the site of pathology along the gastrointestinal tract has important functional significance. The area of bowel principally affected may influence the composition of diarrhoeal fluid, e.g. the considerable bicarbonate losses resulting from small intestinal damage will be reduced if there is no colonic involvement and the reabsorptive capacity of the colon remains intact. Indeed acute infectious diarrhoeas may produce, in the rectal mucosa, changes that are virtually indistinguishable from the appearance of colitis found in chronic inflammatory bowel disease. Granulomata with giant cells have been described in some adults with salmonellosis (McClelland and Gilmour, 1976).
In the past, based on autopsy studies, the pathological findings in children with gastroenteritis have been regarded as non-specific and inconsistent. Giles, Sangster and Smith (1949) described, at post mortem, the pathological features in 49 fatal cases in a childhood epidemic of non-bacterial gastroenteritis. There was mild hyperaemia of the small intestine in 28, submucosal haemorrhages in 13, ulceration of the mucosa in 4, and the small bowel was normal in 4 children.
The rapid autolysis of the surface epithelium of the small intestine, which occurs very soon after death, has in the past greatly hindered critical evaluation of the state of the small intestinal mucosa in children dying from gastroenteritis. In fact, much may be learned from post-mortem studies of the small intestine taken from children who have died following acute gastroenteritis. This is because of the discovery by Creamer and Leppard in 1965 that, although autolysis of the surface epithelium at death is rapid, autolysis of the basement membrane and lamina propria is delayed. By taking advantage of this fact they were able to study the three-dimensional morphology of the small intestine closely along its whole length with a dissecting microscope, once the surface epithelium had sloughed off.
Details of this technique are discussed in Chapter. 3, Ten children who died as a consequence of what proved at autopsy to be enteritis or enterocolitis were studied in this way at the Royal Alexandra Hospital. Death in each case followed the clinical syndrome of acute gastroenteritis after varying intervals. No bacterial pathogen apart from Cl. welchii was found in stools during life. In some children this syndrome accompanied other disease processes, e.g. acute leukaemia, but in others it was the only disease present. Table 6.4 lists the age at death of these 10 children, the diagnosis and the appearances seen under the dissecting microscope in the duodenum, the jejunum, 50 cm proximal to the ileo-caecal valve. The dominant appearance is listed first and any other morphological variant also observed is listed below, roughly quantitated + to + + + .
Table 6.4.
Findings in children with enteritis
| Child | Sex | Age | Diagnosis | Duodenum | Jejunum | Ileum | Ulcers |
|---|---|---|---|---|---|---|---|
| E.M. | M | 3wk | Enteritis | STR | STR | STR | Whole |
| Bronchopneumonia | TR+ + | TR+ + | small intestine | ||||
| F.O. | F | 3wk | Enteritis | TR | T | F | |
| Adrenal haemorrhage | T+ + | — | |||||
| K.F. | F | 6wk | Enterocolitis | STR | STR | STR | Chiefly |
| Chronic pancreatitis Septicaemia Sugar malabsorption | Flat+ | Flat+ | T+ | terminal ileum | |||
| B.W. | M | 2m | Enterocolitis | TR | T | STR | Terminal ileum |
| Pseudomonas and Candida albicans | STR+ + | TR+ + | |||||
| R.S. | M | 7m | Congenital heart disease Enterocolitis | NE | STR | T STR+ | — |
| J.P. | F | 9m | Enteritis | STR+++ | T | T | — |
| TR+ | STR++ TR+ | L+ + | |||||
| R.O. | M | 1 y 4 m | Enteritis | STR | STR | L | — |
| Pulmonary haemorrhage | |||||||
| J.C. | M | 1 y 5 m | Enteritis | STR | TR | T | — |
| Pulmonary oedema | TR+ + | ||||||
| R.C. | M | 5y | Acute leukaemia Enteritis probably due to Cl. welchii | F L+ + | F | F | — |
| M.R. | F | 10 y 4m | Mongolism Diabetes Pancreatic atrophy Enteritis | T | F | F | — |
STR = short thick ridges, TR = thin ridges, T = tongues, L = leaves, NE = not examined, F = fingers.
The presence of short thick ridges (see Figure. 3.28) or a flat mucosa was considered abnormal, as these appearances have been shown to correspond histologically to partial villous atrophy or subtotal villous atrophy, respectively. Long thin ridges, tongues, leaves and fingers were regarded as normal variants.
This study showed that the dissecting microscope appearances within the area surveyed, particularly proximally, showed a variable morphology. When mucosal abnormality was present this was also of variable severity, i.e. the lesion was patchy. In addition, there was a variable pattern of distribution of the mucosal abnormality along the small intestine. In the majority of children the mucosa was most abnormal in the proximal small intestine but in two children the whole length of the small intestine was equally abnormal and in one child the ileum was chiefly affected. The mucosal abnormality occurred more commonly in the children under 6 months of age and the most extensive lesions observed, i.e. involving the whole length of the small intestine, occurred in two of the infants under 6 months.
Figure 6.2, Figure 6.3, Figure 6.4 illustrate diagrammatically the distribution of the dominant mucosal appearances along the length of the small intestine, showing the three patterns, and contrasting them with the morphological findings in a child dying from a non-gastroenterological cause (see Figure. 3.14). Histologically, all children had enteritis with an infiltration of inflammatory cells in the lamina propria, but the three-dimensional morphology ranged in appearance from fingers to a flat mucosa.
Figure 6.2.
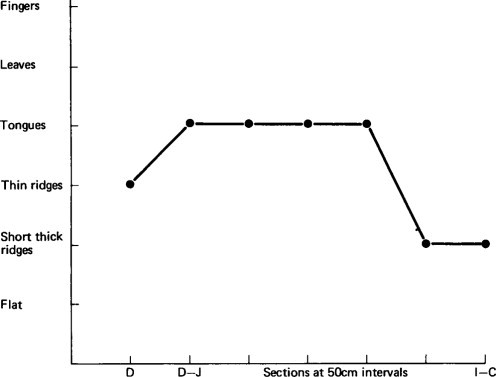
Diagrammatic representation of mucosal appearance along the small intestine in a child dying from gastroenteritis.
Figure 6.3.
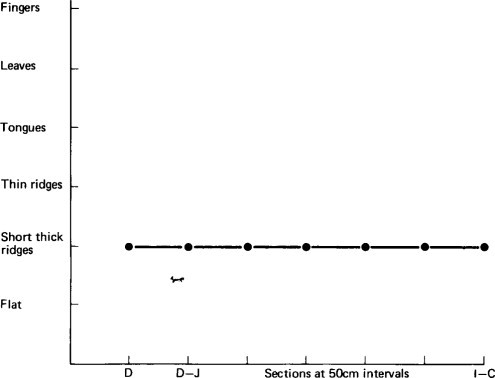
Diagrammatic representation of mucosal appearance along the small intestine in a child dying from gastroenteritis.
Figure 6.4.
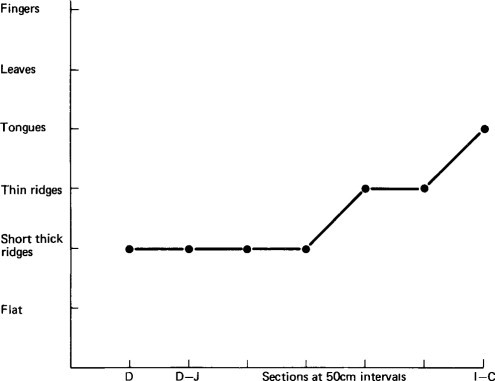
Diagrammatic representation of mucosal appearance along the small intestine in a child dying from gastroenteritis.
The flat mucosa when sectioned histologically (Figure. 6.5 ) had an appearance identical with that seen in children with coeliac disease.
Figure 6.5.
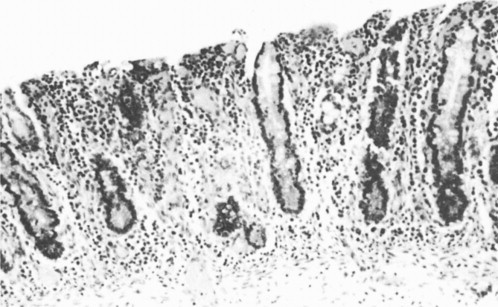
Flat mucosa from a child dying from gastroenteritis.
In these children the most abnormal appearances tended to occur on the tops of the mucosal folds (plicae circulares) or on the edges of mucosal ulcers. In between the mucosal folds the mucosa was less severely abnormal and this difference in morphology between the top of the folds and the valleys between, is shown in Figure. 6.6 . In some children inflammatory ulcers were seen with varying patterns of distribution along the small intestine.
Figure 6.6.
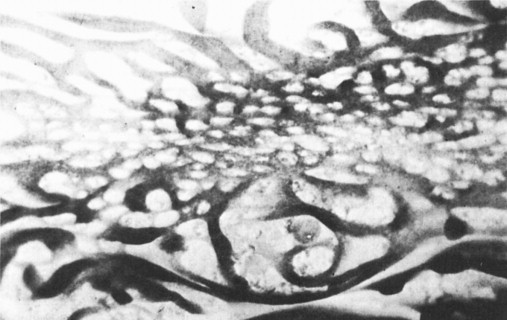
Dissecting microscope appearances showing flat mucosa on top of mucosal folds with ridges on the side of the folds.
From this study it may be concluded:
-
•
The mucosal damage which occurs as a consequence of gastroenteritis is of variable severity in the proximal small intestine, i.e. it is a patchy lesion.
-
•
That the pattern of distribution of this mucosal abnormality along the small intestine is very variable.
-
•
That ulceration of the small intestine is relatively common.
This study was undertaken before rota virus had been discovered and the cause of the gastroenteritis in these children is thus unknown.
It may be inferred that a single proximal small intestinal mucosal biopsy could not be interpreted as reflecting the state of the whole small intestinal mucosa along its length, nor may it reflect accurately even the overall state of the mucosa in the region of the small intestine biopsied in these children.
These observations raise difficulties in interpretation of both single and serial biopsies. Proximal biopsies may be taken most often from the more accessible exposed tops of mucosal folds rather than the more inaccessible valleys in between. Serial biopsies may thus tend to be taken from roughly the same area, but this is pure speculation. This study does suggest that multiple biopsies would reflect more accurately than single biopsies the true state of the small intestinal mucosa.
Barnes and Townley (1973) used single small intestinal biopsies to investigate the state of the duodenal mucosa in 31 infants with acute gastroenteritis. Their study confirmed the observations that a mucosal lesion similar to that seen in coeliac disease may occur in children who have gastroenteritis, as five of these infants had such a lesion on biopsy. Only five infants had normal small intestinal biopsies, and in the remaining infants mild (11) or moderate (10) mucosal abnormality was present. In three patients, serial biopsies after 3 days, 8 days and 7 weeks showed significant improvement.
Schreiber, Blacklow and Trier (1975) in the USA have studied the effect of oral administration of a stool filtrate containing the Norwalk agent upon the small intestinal mucosa in 15 adult volunteers. All the volunteers had normal baseline small intestinal biopsies; 12 developed clinical gastroenteritis and an abnormal small intestinal mucosa with villous shortening, crypt hypertrophy and mucosal inflammation. One of the three asymptomatic volunteers also developed mucosal abnormality. Biopsies 6–8 weeks later were normal. These authors used a multiple biopsy technique. In some cases, when two simultaneous biopsies were taken, the severity of the mucosal abnormality varied and it was concluded that the mucosal lesion involving the proximal small intestine could be patchy, thus supporting the observations made in the above childhood autopsy study. They postulated that the Norwalk agent initially damages the villous absorptive cell, causing acute inflammation. Then, probably as a compensation, the crypt hypertrophy and epithelial cell proliferation occurs to replace the damaged enterocytes.
Mortality
Before the development of modern methods of preventive medicine and of intravenous therapy, gastroenteritis had a very high mortality in the Western world (Figure. 6.7 ). This has been substantially indeed dramatically reduced but in developing countries, as Kretchmer (1969) has pointed out, a child under the age of 7 years still has a 50% chance of dying from a diarrhoeal disease. Gastroenteritis thus remains a factor of the greatest importance in the continuance of a high death rate in infancy and early childhood in many developing communities (Rohde and Northrup, 1976). The annual number of cases of acute diarrhoeal deaths in 1980 for children under 5 years of age has been estimated for Africa, Asia (excluding China) and Latin America (Snyder and Merson, 1982). This proved to be 4.6 million diarrhoeal deaths per year, a gigantic figure.
Figure 6.7.
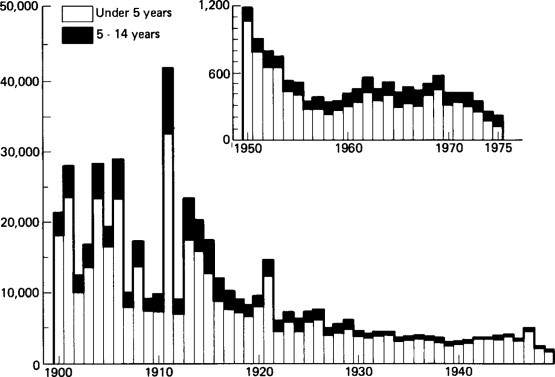
Annual deaths from diarrhoeal disease in England and Wales 1900–1975.
Spencer and Coster in Johannesburg (1969) have shown a striking fall in mortality rate from gastroenteritis in African children, in the age group 0–11 months, during the 10-year period 1956–1966. They attributed this improvement chiefly to betterment of socio-economic and environmental circumstances rather than to improved medical services and therapeutic techniques. However, such improvements do not lead to a disappearance of gastroenteritis from the community but may in fact alter its aetiology and, also, its clinical severity.
In Sydney, in 1970, at the Royal Alexandra Hospital, there were six deaths from gastroenteritis out of 610 public admissions to the gastroenteritis unit, i.e. a mortality of 0.9%. This has now (1987) fallen to zero.
The mortality rate for children admitted with acute gastroenteritis to the Queen Elizabeth Hospital in 1973, was 0.6% (Gribbin, Walker-Smith and Wood, 1976). This fell to zero in 1979 and there have been no in-patient deaths in the hospital from gastroenteritis per se since then.
This recent fall is mirrored in the national statistics (Figure. 6.8 ) accounted for by the virtual disappearance of hypernatraemia complicating gastroenteritis as a cause of death. The persistence of mortality from acute gastroenteritis in the 1970s was in large measure related to the occurrence of hypernatraemia at that time. This is discussed later.
Figure 6.8.
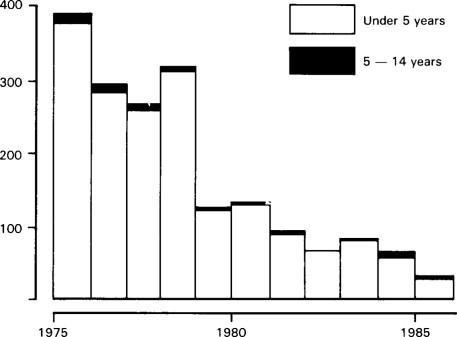
Annual deaths from diarrhoeal disease in England and Wales 1975–1985.
In England and Wales, in 1970, 411 children under 15 years of age were reported to have died from this disorder. A Department of Health and Social Security survey in the United Kingdom of three health areas for 3 years (1964–1967) revealed 679 post-natal deaths of infants aged 1 month to 1 year; 77 (11%) of these were from gastroenteritis. The Department of Health and Social Security survey indicated that some of the deaths were sudden and unexpected. Sometimes infants who were apparently making good progress under treatment collapsed and died. In other infants, the onset of the illness was sudden and its course so fulminant that, even though they were brought quickly to hospital, they were moribund on admission. In only 11 of 77 deaths was it clear that progressive deterioration over a period of days had occurred at home. In some of these earlier cases specialist aid may have reduced the mortality but, in general, the unpredictability of the severity of this disorder in Britain made it hard to design measures to further reduce its mortality.
In any event the current fall in mortality in the early 1980s represents a further large and dramatic fall from the 1970s not predicted by this report. A brief word should be made about the current mortality statistics. These are based upon a classification now out-dated by modern knowledge of the cause of gastroenteritis and it is difficult to collect the global figure for all children dying from gastroenteritis. Clearly, revision is urgently required (see Figures. 6.7 and Figures. 6.8).
This fall in mortality (especially in infancy) from gastroenteritis now places the United Kingdom on a comparable footing with other countries of Europe whereas in the 1970s Britain had a higher mortality than many other European countries. WHO World Statistics Annual Geneva 1986: Individual Tables reports the infant mortality attributable to gastroenteritis in 1983 as 6 per 100 000 for England and Wales, 6 for Scotland and 0 for Northern Ireland. Similar figures come from France (6), West Germany (3) and Netherlands (0), whereas there are much higher figures e.g. 113 for Portugal. The advent of low solute milks and the widespread use of glucose electrolyte solutions probably account for this fall as well as less tangible improvements in living conditions.
Susceptibility to gastroenteritis
Children under the age of 2 years are more susceptible to this infection than older children in whom infection is not common except in outbreaks within a family or institution. Malnourished children also have a greater susceptibility than normal children.
It has long been known that breast feeding reduces the chance of an infant developing gastroenteritis but it does not abolish such a risk entirely, as documented by Kingston (1973) in West Africa. It is true, however, that gastroenteritis is uncommon in infants who are exclusively breast fed. Various reasons for this have been put forward, several of which have already been mentioned in Chapter. 1 (see page 20).
A word of caution must be made about the preventative role of breast feeding (Mittal, 1986). Breast feeding consistently has been shown to provide protection against gastroenteritis as compared with bottle-fed infants but the incidence of diarrhoeal diseases even amongst breast fed infants in the more deprived parts of the world is still much higher than for bottle-fed infants living in developed communities. Bacterial and also rotavirus infections can occur in breast-fed infants. In areas of socioeconomic deprivation and heavy environmental contamination decreased protection may be due to deficient cellular and immune factors in the breast milk of malnourished mothers.
There has been discussion concerning the relative importance of specific and non-specific antimicrobial factors transferred in breast milk to the infant's gut. It seems probablethat both are important (Soothill, 1976). Specifically, the IgA and lactoferrin content of breast milk (Bullen and Willis, 1971) are of particular importance for protection against enteropathogenic strains of E. coli. Robinson, Harvey and Soothill (1978) have also shown that macrophages and neutrophils from human milk phagocytose and kill E. coli in vitro after opsonization by the aqueous phase of milk. This is likely to be an important protective mechanism in vivo.
The protective role breast milk affords the suckling infant against the hazards of enteropathogenic E. coli enteritis have been dramatically demonstrated by Stoliar et al. (1976). These workers took colostrum from Guatemalan mothers 2-4 days postpartum and breast milk from both Guatemalan and North American mothers and found that both their milks inhibited the fluid accumulation in rabbit ileal loops that was induced when the loops were incubated with both E. coli enterotoxin and cholera enterotoxin. Furthermore, it was shown that the antitoxin activity of the mothers’ milk correlated with its IgA content but not its IgG or IgM content. This provides vivid and clear evidence of the protective role breast feeding may provide.
It has, however, already been made clear that the protection breast feeding gives against gastroenteritis is not complete. Protection is diminished when the infant is being weaned onto solid food or cows' milk, i.e. when the baby is not exclusively breast fed. The risk is obviously far greater when these weaning foods are heavily bacterially contaminated. The problem posed by this in the developing world has been highlighted by Rowland, Barnell and Whitehead (1978) who have pointed out that traditional weaning foods used for young infants in West Africa can be hazardous bacteriologically. Thus providing a breast-fed infant with such supplements under the prevailing conditions in the developing world may be dangerous. Indeed, because of such weaning practices with bacteriologically contaminated food, infantile infective diarrhoea can continue to be very common in a community, despite breast feeding. Commercial baby milks and feeding bottles in such an environment can also carry similar risks. In such traditional societies, however, infants remain relatively free from gastrointestinal disorders so long as they are exclusively breast fed.
Hence, the infant in the developing world is most at risk during the transition from breast feeding to a full diet. In such communities, all food babies ingest, except breast milk, must be regarded as being highly contaminated potentially, and so a cause of weanling's diarrhoea. Jelliffe and Jelliffe (1978) suggest that the situation can be helped if attempts are made to improve lactation in mothers by improving their nutrition, by persuading parents to delay introducing weaning foods to babies until the age of 4–6 months, by making the weaning foods more nutritious, and by using easy-to-clean plastic or metal feeding containers avoiding contamination.
Prevalence and seasonal variation
The peak prevalence of gastroenteritis in infancy is related to the age of weaning. In most countries in the developing world, this means that peak prevalence occurs in the second year of life, which corresponds to the peak prevalence in protein energy malnutrition. By contrast, in developed communities the peak prevalence occurs during the first year of life. This was the pattern observed during 1973, in the study of Gribbin, Walker-Smith and Wood (1976a b c)) with the largest group of admissions under 6 months of age (see Figure. 6.27 ). More recently at the Queen Elizabeth Hospital, there has been a trend for children of more than 6 months to be admitted with acute gastroenteritis. This runs parallel with the trend for more breast feeding in the community.
Figure 6.27.
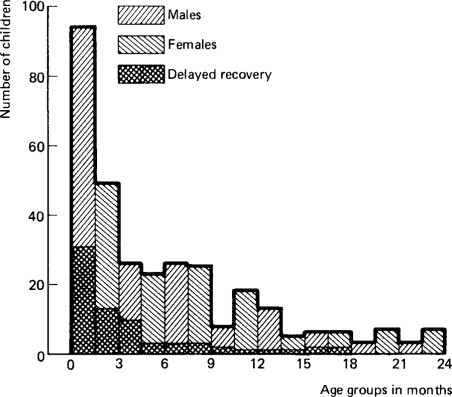
Age and sex of children with delayed recovery after gastroenteritis under 2 years.
Gastroenteritis continues to be a problem of world-wide importance as the following facts indicate. Approximately 500 million episodes of diarrhoea have been estimated to occur in children under 5 years in Asia, Africa and Latin America in 1975 (Rohde and Northrup, 1975). Thus, gastroenteritis continues to be a problem of enormous importance in developing communities; for example, Papua New Guinea, where gastroenteritis was the commonest cause of hospital admission in children aged 1–4 years in 1967 (Biddulph and Pangkatana, 1971). Its prevalence is a major factor in the genesis of protein-calorie malnutrition in such communities. In South Africa, Hansen (1968) has observed in a careful 1-year follow-up of 80 admitted to hospital with gastroenteritis in Capetown, that 12 subsequently developed kwashiorkor, 37 had loss of weight and failed to improve during the year of observation, and a total of 62 children had recurrent diarrhoea of varying severity. Walker (1971) has estimated that 20% of Australian aboriginal children in the northern part of the Northern Territory are admitted to hospital with gastroenteritis before their second birthday. Thus there is a close interaction between malnutrition, gastroenteritis and chronic diarrhoea in developing communities, and gastroenteritis continues to be a very important health hazard among children from such communities.
In Western societies gastroenteritis continues to be a common problem. The total number of children admitted each year to the gastroenteritis unit at the Queen Elizabeth Hospital over the past 25 years tended to rise until the early 1970s (Figure. 6.9 ), although the number of children who required intravenous fluids had at the same time fallen, i.e. the indications for admission to the unit had changed with many more children in recent years being admitted for social reasons. Through the 1970s the number of admissions was fairly constant, but has fallen significantly in the 1980s. However, there does continue to be a daily outpatient gastroenteritis clinic. Figure. 6.10 shows the number of children admitted each week to the gastroenteritis ward during 1970 at the Royal Alexandra Hospital. The annual total of admissions for that year was 838.
Figure 6.9.
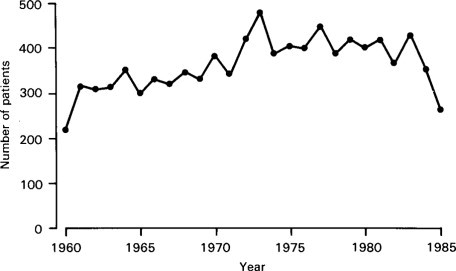
Annual admissions to gastroenteritis unit, Queen Elizabeth Hospital 1960-1985.
Figure 6.10.
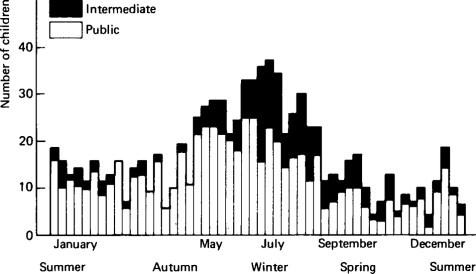
Number of weekly admissions in the gastroenteritis unit at the Royal Alexandra Hospital for 1970. Black represents ‘intermediate patients’ and white ‘public patients’.
Thus it is still true that gastroenteritis continues to be an important problem in developed countries, although its dimensions are not as great as in the developing nations.
Gastroenteritis used to be considered an epidemic summer disease in countries such as Britain, the United States and Australia. Figure. 6.11 shows that in Sydney it is now a disease with a winter peak (Walker-Smith, 1972). Such a trend to an increased prevalence in winter has also been reported by Moffet, Shulenberger and Burkholder (1968) and others from the United States. Ironside (1973) in Britain has also reported a similar trend in Manchester, and it has also been observed at the Queen Elizabeth Hospital in London for rotavirus (Figure. 6.12 ). In contrast, Spencer and Coster (1969) in South Africa have shown that gastroenteritis is still a predominantly summer disease among the African population.
Figure 6.11.
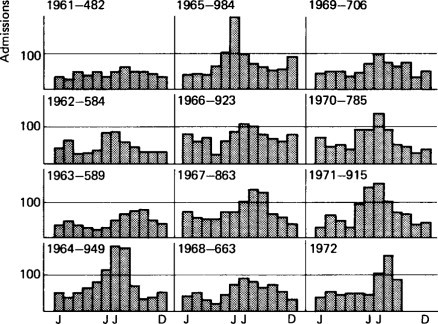
Monthly admissions each year to the gastroenteritis unit at the Royal Alexandra Hospital 1961-1972.
Figure 6.12.
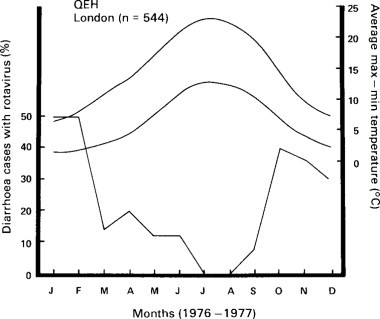
Percentage of children with acute diarrhoea at Queen Elizabeth Hospital who had rotavirus in stool, related to seasons. Top two curves temperature, bottom percentages of cases with rotavirus.
Dorman (1972) has studied the monthly number of admissions to the gastroenteritis unit at the Royal Alexandra Hospital over a 10-year period and has shown that the winter peak appeared for the first time in 1964, and was associated with a large increase in the total number of annual admissions. This pattern of admissions to the unit has continued thereafter (see Figure. 6.11). During this winter peak, most patients are infants and the percentage of bacterial isolations often falls as low as 2-5%. These observations suggest that a new, probably viral, pathogen may have appeared in Sydney in 1964, altering the pattern of gastroenteritis in the community. This is likely to have been the rotavirus.
Gastroenteritis is often associated with poor housing, overcrowding and low standards of hygiene, but the Sydney survey in 1970 showed that many of the patients during the winter months were ‘intermediate patients’ (see Figure. 6.11), i.e. children from the upper socioeconomic groups. A geographical survey of Sydney showed that children with gastroenteritis came from all sections of the community, rich and poor alike. Thus it must be assumed that the mere improvement in living standards will not lead automatically to a disappearance of gastroenteritis. Such measures may simply result in a change in its aetiology.
However, it does seem clear now that gastroenteritis has a very low prevalence in rural areas in countries such as Britain. Crowding, a condition of urban life, may be a factor in the continuance of gastroenteritis in children of large, developed cities. Although rotavirus has not been found yet in throat washings, etc., Reiman, Hodges and Price (1945) showed that inhalation of nebulized filtered throat washings from patients with acute gastroenteritis were capable of producing disease in volunteers. Urban spread of gastroenteritis therefore may be related to droplet infection rather than to the ano-oral route alone. A ‘faecal aerosol’ may be one important mode of spread.
Clinical features and differential diagnosis
As the definition of gastroenteritis makes clear, it is a disease characterized by the acute onset of vomiting and diarrhoea. A summary of the clinical features of the different types of acute gastroenteritis are listed in Table 6.5 . It is helpful to divide the pattern of acute diarrhoea into acute watery diarrhoea, dysenteric diarrhoea and prolonged diarrhoea beginning acutely but which has lasted for 7 days or more but short of 2 weeks. More than 2 weeks would be the postenteritis syndrome. This is perhaps somewhat over-simplified as blood may be present in the acute watery stools especially of infections with salmonellae and Campylobacter. Amoebiasis causes a colitis and thus is strictly outside the terms of reference of this book. Focusing on most frequently seen syndromes, Table 6.6 illustrates their principal features.
Table 6.5.
Clinical features of acute diarrhoeal disease useful in differential diagnosis
| Clinical features | Shigella | Enteropatho-genic E. coli | Salmonella (excluding typhoid fever) | Non-bacterial |
|---|---|---|---|---|
| Age | 6 m−5 y (rare in neonate) | Less than 2 y | Any age | Any age |
| Diarrhoea in household | Common (>50%) | No | Variable | Variable |
| Onset | Abrupt | Gradual | Variable | Abrupt |
| Vomiting as a prominent symptom | Absent | Uncommon | Common | Common |
| Fever (over 39°C, 102°F) | Common | Absent | Variable | Uncommon |
| Respiratory symptoms | Common (bronchitis) | Absent | Uncommon (except in septicaemic form) | Common (upper respiratory) |
| Convulsion | Common | Rare | Rare | Rare |
| Anal sphincter | Lax tone (rarely, rectal prolapse) | Normal | Normal | Normal |
| Time after onset when seen by doctor | Early | Several days | Several days | Early |
| Early course, untreated | Slight or no improvement | Persistent or | Persistent | Daily improvement |
Reproduced from Nelson and Haltalin (1971) by kind permission of authors and publishers.
© 1988
Table 6.6.
Most common syndromes
| Aetiological agents |
Clinical features |
Incubation period | Epidemiological features | Firstline treatment | ||
|---|---|---|---|---|---|---|
| Common | Others | |||||
| Rotavirus | Vomiting | Fever. Severe dehydration in some | 24-72 hours | Infants and young children particularly. Common world-wide in all socio-economic groups. Peak in winter in temperature climates | Oral rehydration therapy | |
| Acute watery diarrhoea | Enterotoxigenic E. coli | Nausea. Vomiting | Fever. Malaise. Severe dehydration | 6–72 hours | Infants and young children in developing countries. Travellers' diarrhoea | Oral rehydration therapy |
| Enteropathogenic E. coli | Nausea. Vomiting | Fever | 6–72 hours | Nursery outbreaks in developed countries. Children in developed countries. Uncertain in developing countries. | Oral rehydration therapy | |
| Non-typhoid Salmonellae | Nausea. Vomiting. Fever. Chills. Abdominal pain | Malaise | 8–36 hours | Children. Common world-wide. Food-borne outbreaks (animal products, e.g. chicken meat) Warmer seasons | Oral rehydration therapy | |
| Campylobacter | Abdominal pain. Malaise | Chills stools | 3–5 days | World-wide distribution. In developed countries may be transmitted by handling of animals | Oral rehydration therapy in severe cases | |
| Vibrio cholerae | Vomiting. Abdominal pain | Severe dehydration. Circulatory collapse ‘shock’ | 1–3 days | Children in endemic areas. Adults in newly affected areas. Not found in Latin America. | Oral rehydration therapy. Tetracycline | |
| Dysentery. Stool is soft and watery with blood and/or pus | Shigellae | Fever. Abdominal pain | Malaise. Vomiting. Urgency to defaecate. Painful spasm on defecation | 36–72 hours | Children. Poor hygiene. Malnutrition. Instititions. Warmer-seasons | Oral rehydration therapy Trimethoprimsulfamethoxazole |
| Prolonged diarrhoea (7–14 days) | Entamoeba histolytica | Abdominal discomfort | 2–6 weeks | All age groups. World-wide distribution | Metronidazole | |
| For at least 7 days stools have been more frequent or of softer consistency (with or without blood or pus) | Giardia lamblia | Abdominal distension. Flatulence | Anorexia. Nausea. Malabsorption. Frothy stools | 1–3 weeks | Young children. Some travellers. Poor hygiene. World-wide distribution | Metronidazole |
When a child with acute vomiting and diarrhoea is first seen, the clinician's initial step in his assessment is to determine, on clinical grounds, whether the child has gastroenteritis or whether there is evidence that he has some other disease state that may manifest in a similar way. Table 6.7 lists some of the disorders that may present with acute vomiting and diarrhoea, with features that may suggest the diagnosis of acute gastroenteritis.
Table 6.7.
Illnesses that can cause symptoms of diarrhoea and vomiting that may be confused with gastroenteritis
| Surgical disorders: | Coeliac disease |
| Acute appendicitis | Systemic infections: |
| Intussusception | Septicaemia |
| Pyloric stenosis | Pneumonia |
| Various causes of incomplete | Meningitis |
| intestinal obstruction, | Urinary tract infection |
| including Hirschsprung's | Measles (especially if malnourished) |
| disease | Malaria |
| Inflammatory bowel disease: | Upper respiratory tract infections |
| Necrotizing or non-specific | Otitis media |
| Enterocolitis | |
| Crohn's disease | |
| Ulcerative colitis | |
| Acute food intolerance | Other disorders |
| e.g. cows' milk protein intolerance | Diabetic pre-coma |
| Haemolytic–uraemic syndrome | |
| Haemorrhagic shock and | |
| encephalopathy* |
Levine et al. (1983).
Some of the disorders listed are relatively trivial, e.g. upper respiratory tract infection. The mechanism of diarrhoea and vomiting found in such infants is not clear and the illness resulting is usually mild. The author prefers to avoid the term parenteral diarrhoea because we need to look carefully at this group. It is now clear that a child with otitis media and acute diarrhoea may have a rotavirus gastroenteritis (Rodriquez et al., 1977). It is also probable that adenovirus can cause both an upper respiratory tract infection and gastroenteritis.
Other conditions in this list are far more serious, and failure to make the correct diagnosis may have dire consequences for the child. An acute abdomen is the greatest risk, and those paediatricians who care for a gastroenteritis unit are evermindful of the risk of failing to diagnose an infant with a surgical condition admitted to their unit. A detailed history carefully taken, coupled with a simple yet thorough clinical examination can usually exclude most of the conditions listed or at least indicate the appropriate investigations that need to be done immediately. These may include simple ward testing of urine, urine culture or a blood culture.
Danger signs on physical examination include abdominal distension, abdominal tenderness and a degree of ‘toxicity’ out of proportion to the child's state of hydration. Abdominal distension or tenderness suggest the possibility of an acute abdomen and a plain X ray is always indicated. Sometimes gastroenteritis per se may cause such abdominal distension with a dilated gut. None the less, such infants should be observed very carefully, even when there is no evidence of obstruction on X-ray of the abdomen.
Bloody diarrhoea, although it is a frequent accompaniment of bacterial gastroenteritis, should also alert the clinician to the possibility of intussusception and the haemolytic-uraemic syndrome in the small child, as well as ulcerative colitis and Crohn's disease more often in the older child.
The second step in clinical assessment of a child with gastroenteritis, once the diagnosis has been made, is to assess the child's state of hydration. The clinical signs and a rough clinical assessment of the extent of the dehydration are listed in Table 6.8 . When a child is assessed as 5% or more dehydrated he may require intravenous fluids and so should be admitted to hospital, at least in developed countries. However, this is not the only reason for hospital admission in a child with gastroenteritis. Other possible reasons are listed below:
-
•
Children requiring intravenous therapy, i.e. 5% or more dehydration.
-
•
Failure of outpatient management.
-
•
Uncertainty about the state of hydration, e.g. obese infants.
-
•
Uncertainty about diagnosis, especially if a surgical lesion is expected.
-
•
Children who have a severe history although not yet dehydrated.
-
•
Social circumstances.
Table 6.8.
Clinical signs and assessment of extent of dehydration
| I Signs of dehydration | Practical guide: RAHC and QEHC |
|---|---|
| 2–3% dehydration: | Thirst, mild oliguria |
| 5% dehydration: | Discernible alteration in skin tone, slightly sunken eyes, some loss of intraocular tension, thirst, oliguria. Sunken fontanelle in infants |
| 7-8% dehydration: | Very obvious loss of skin tone and tissue turgor, sunken eyes, loss of intraocular tension, marked thirst and oliguria. Often some restlessness or apathy. |
| 10% dehydration (and over): | All the foregoing, plus peripheral vasoconstriction, hypotension, cyanosis, and sometimes hyperpyrexia. Thirst may be lost at this stage. |
| II Assessment of dehydration and fluid deficit (WHO) | |||
|---|---|---|---|
| Signs and symptoms | Mild dehydration | Moderate dehydration | Severe dehydration |
| General appearance and condition | |||
| Infants and young children | Thirsty; alert; restless | Thirsty; restless; or lethargic but irritable when touched | Drowsy; limp; cold; sweaty, cyanotic extremities; may be comatose |
| Older children and adults | Thirsty; alert; restless | Thirsty; alert; giddiness with postural changes | Usually conscious; apprehensive; cold, sweaty cyanotic extremities, wrinkled skin of fingers and toes; muscle cramps. |
| Radial pulse1 | Normal rate and volume | Rapid and weak | Rapid, feeble, sometimes impalpable |
| Respiration | Normal | Deep, may be rapid | Deep and rapid |
| * Anterior fontanelle2 | Normal | Sunken | Very sunken |
| * Systolic blood pressure3 | Normal | Normal-low | Less than 10.7 kPa (80 mm Hg); may be unrecordable |
| *Skin elasticity1 | Pinch retracts immediately | Pinch retracts slowly | Pinch retracts very slowly (> 2 seconds) |
| *Eyes | Normal | Sunken | Deeply sunken |
| Tears | Present | Absent | Absent |
| Mucous membranes5 | Moist | Dry | Very dry |
| *Urine flow6 | Normal | Reduced amount and dark | None passed for several hours; empty bladder |
| Body weight loss (%) | 4–5 | 6–9 | 10% or more |
| Estimated fluid deficit | 40–50 ml kg−1 | 60–90 ml kg−1 | 100–110 ml kg−1 |
4 Not useful in marasinic malnutrition or obesity.
If radial pulse cannot be felt, listen to heart with stethoscope.
Useful in infants until fontanelle closes at 6–18 months of age. After closure there is a slight depression in some children.
Difficult to assess in infants.
Dryness of mouth can be palpated with a clean finger. Mouth may always be dry in a child who habitually breathes by mouth. Mouth may be wet in a dehydrated patient due to vomiting or drinking.
A marasmic baby or one receiving hypotonic fluids may pass good urine volumes in the presence of dehydration.
RAHC = Royal Alexandra Hospital for Children.
QEHC = Queen Elizabeth Hospital for Children.
Particularly useful in infants for assessment of dehydration and monitoring of rehydration.
In fact failure of outpatient management may often be for social reasons, which has been a common cause in both the Royal Alexandra Hospital and the Queen Elizabeth Hospital.
In 1973, Gribbin, Walker-Smith and Wood (1976a b c) made a prospective study of the clinical features of all children admitted to the gastroenteritis unit at the Queen Elizabeth Hospital with provisional diagnosis of acute gastroenteritis. There were 472 admissions, accounting for 6.8% of the total admissions to the hospital in that year. Readmissions for recurrence of symptoms or for social problems were required for 45 of the 416 children admitted that year. Their ages ranged from 2 days to 8 years 10 months, but the majority were under the age of a year, the largest number being under 6 months. There was a clear male predominance in the youngest age group; 64% of infants under 3 months were boys.
In 24 children the primary diagnosis proved in fact after admission not to include gastroenteritis. Two children were found to have coeliac disease.
This study clearly illustrates the diversity of causes for the syndrome of acute gastroenteritis. At that time, unfortunately, stool electron microscopy was unavailable and so rotavirus was not identified. Of the aetiological agents recognized in that year, only 15% had a bacterial pathogen from the total of 427 admissions.
Only 9 of 416 admissions were breast fed and of these only one infant fully breast fed. At that time the bottle-fed infants were being fed principally with high solute milks, and cereals were often introduced inappropriately early. 14% of those diagnosed as acute gastroenteritis whose plasma sodium was estimated within 4 hours of admission proved to have hypernatraemia. The situation has now completely changed, with very few cases of hypernatraemia.
Water and electrolyte disturbances in gastroenteritis
The most important acute complication of gastroenteritis is dehydration which occurs when the child's overall output of fluid exceeds input. As he becomes dehydrated, the volume and the electrolyte concentration of his intake usually become the primary determinants of the final state of the body's fluid tonicity, i.e. whether the dehydration is hypertonic, isotonic or hypotonic, but an increase of insensible water loss due to fever, hyperventilation, or a dry environment may also play a role in influencing such tonicity.
Insensible water loss is the volume of fluid that leaves the body as a result of the difference in vapour pressure between the skin and lung surfaces and the surrounding atmosphere. Plasma osmolality is normally 275–295 mosmol kg−1.
The stool fluid losses in children with gastroenteritis are typically hypotonic, i.e. there is more than 1 1 water per 150 mmol of Na+ in the stools. Weil (1973) has emphasized that the actual tonicity of the extracellular fluid is determined most importantly, not by stool losses but by the volume and tonicity of the child's intake, although the ability of the kidney to excrete hypertonic urine is also an important determinant. In general, the tonicity of the child's intake is due to its content of salt.
Weil has described the sequence of events that occur in relation to fluid tonicity in infants with gastroenteritis who become dehydrated. Three of his examples will be briefly summarized since they help our understanding of the mechanisms that cause infants to develop dehydration of varying tonicity.
First, when no intake occurs the extracellular fluid tends to become hypertonic, but the kidneys can correct this by excreting hypertonic urine to restore isotonicity. There is also some movement of fluid from the intracellular fluid to the extracellular as a result of the osmotic gradient. However, as fluid loss continues the plasma volume falls and renal function begins to fail, and when this occurs the extracellular fluid becomes hypertonic.
Secondly, when there is a small intake of water and some sodium, the small intake acts as if the size of the insensible water loss is reduced but the Na+ present adds to the work of the kidneys which makes hypertonic dehydration more likely.
Thirdly, when there is a large fluid intake that is essentially water or very dilute Na+ containing, the end-result is hypotonic dehydration, because the net loss of Na+ is in excess of the net loss of water.
Weil has concluded that the ideal time for initiating therapy for infants with gastroenteritis is when the intake of fluid is so balanced that an initial volume of fluid equivalent to the insensible water loss can be given in an essentially Na+ free form. Any fluid over that volume will then contain a moderate amount of Na+ and K+ with a concentration approaching that in the diarrhoeal stool.
The importance of the volume and salt content of the infant's feeding in the genesis of the type of dehydration produced cannot be over-emphasized. It is clear that although a healthy infant's kidney can deal with a high solute load by producing more concentrated urine, high solute loads in the presence of gastroenteritis will lead to the kidney being unable to cope, resulting in hypertonic dehydration. Indeed, Colle, Aroub and Raile (1958) have shown that babies given feedings with high levels of sodium were, when they had gastroenteritis, more likely to develop hypertonic dehydration. Oates (1973) has shown in a study of 100 mothers of infants under 6 months of age in London, that 22 were making up the infant's milk formula high solute in a more concentrated strength than that recommended. This was due to the use of heaped or packed scoops instead of level scoops of milk powder, or even by using extra scoops. This risk can be significantly reduced by maternal education concerning the risk of using over-concentrated feeds, the use of low solute milk formulae, and the promotion of breast feeding.
Hypernatraemia dehydration
Hypernatraemia is defined as a concentration of sodium in the serum of 150 mmol 1−1 or greater.
This type of dehydration will be discussed in more detail as it has a higher mortality than the other types of dehydration. In addition, clinically significant damage to the central nervous system occurs more commonly and there are more hazards involved in its management. Finberg in the USA in 1970 found that in his experience isonatraemic dehydration accounted for about 65% of dehydrated infants with gastroenteritis, hypernatraemia for 20-25%, and hyponatraemia for approximately 10%. However, in 1973 he found that the frequency of hypernatraemic dehydration had risen to 28–40% of the annual number of admissions of infants with gastroenteritis seen in New York.
In London, hypernatraemia was found in only 14% of children with acute gastroenteritis at the Queen Elizabeth Hospital in 1973. In fact, following publication of the Department of Health and Social Security's Report Present-day Practice in Infant Feeding in 1974, and revised subsequently, which described the hazards of high solute milks, there has been a remarkable fall in the prevalence of hypernatraemic dehydration. This change relates to the replacement of high-solute milks by low-solute milks for most infants under 6 months in Britain. The only way an infant can have high solute milk today is if he is given undiluted cows' milk. Manuel and Walker-Smith (1980) observed a fall in the number of children admitted with hypernatraemic dehydration to the gastroenteritis unit at the Queen Elizabeth Hospital, and this trend has since accelerated. This change is illustrated in Table 6.9 . It is noteworthy that there were deaths in the early years but none since 1977. Indeed the disappearance of hypernatraemic dehydration as a cause of death has been reported in Sheffield, from December 1976 (Sunderland and Emery, 1979) and in the Royal Hospital for Sick Children, Glasgow, from 1977 (Arneil and Chin, 1979). The relative importance of warnings concerning the overconcentration of feeds and the switch from high-solute to low-solute milks leading to this change is emphasized differently by the two groups. Whichever factor is more important it is clear that reduction in the solute load fed to infants in Britain has dramatically reduced the incidence of hypernatraemic dehydration and has been a major factor in further lowering infant mortality from gastroenteritis (Davies et al., 1977).
Table 6.9.
Incidence of hypernatraemia in gastroenteritis in four 12-month periods at Queen Elizabeth Hospital
| 1973 | 1975–6 | 7977 | 1978–9 | |
|---|---|---|---|---|
| Total with gastroenteritis | 472 | 434 | 530 | 507 |
| Infants with hypernatraemia | 23(5%) | 11(2.5%) | 5(0.9%) | 4(0.7%) |
| Infants with Na 160 mmol I−1 or more | 8(1.7%) | 3(0.7%) | 1(0.2%) | 0 |
| Deaths | 2 | 1 | 1 | 0 |
In this type of dehydration there are two physiological disturbances present, namely a loss of body water and a maldistribution of water between the major compartments of body water. This variety of dehydration is sometimes incorrectly equated with hyperosmolar dehydration, but although hypernatraemia is the most frequent cause of hyperosmolality in infants, high blood glucose levels, for example, may also be the cause, and hyperosmolar dehydration may occur in diabetes mellitus without hypernatraemia. Some of the factors predisposing to hypernatraemic dehydration are listed in Table 6.10 .
Table 6.10.
Factors predisposing to hypernatraemic dehydration
| Prematurity | Small mass relative to surface area |
| Immaturity of renal function | |
| Increased insensible water loss | Prolonged fever hyperventilation |
| Hyperventilation | |
| Salicylate toxicity | |
| Low humidity | |
| High-solute load in feeding | |
| Cessation of intake |
Finberg (1973) has reviewed hypernatraemic dehydration in detail and has observed that the relative preservation of the circulation and the early presence of neurological symptoms are hallmarks of the disorder. The volume of the extracellular fluid tends to be maintained to a greater degree in hypertonic dehydration when compared with isotonic dehydration and so signs of circulatory collapse appear later. Their appearance is ominous because of the degree of water deficit by then apparent. In addition, the usual skin criteria for diagnosing dehydration are not accurate, the skin having a characteristic doughy consistency. The fontanelle is typically not sunken, and in many cases may even bulge. This type of dehydration tends to occur more often in obese infants, being related to previous high solute intake. There is a risk in such infants that the signs of dehydration may be underestimated because of their obesity. The state of consciousness is usually depressed, and some infants with a severe degree of hypernatraemic dehydration may have convulsions, which may be related to brain shrinkage, a drop in CSF pressure causing haemorrhage in the central nervous system. Cerebral thromboses may also sometimes occur.
Renal tubular necrosis and hyocalcaemic tetany are less common complications but hyperglycaemia may often accompany hypernatraemia and may lead to an incorrect diagnosis of diabetes mellitus being made. Such a phenomenon is transient and tends to occur when there is coexistent severe acidosis. Insulin should not be given because lowering of blood glucose levels may enhance the risks of developing cerebral oedema during treatment, as correction of the fluid and electrolyte balance may be too rapid.
Hyponatraemia and hypokalaemia
These biochemical findings are more common in those infants who are already severely malnourished when they develop gastroenteritis. It is known that there is a predisposition for such infants to develop hypotonic dehydration. Abdominal distension and absent or markedly diminished bowel sounds strongly suggest K+ depletion.
It is important to realize that the aetiology of gastroenteritis and the water and electrolyte disturbances that occur have a profound geographical variation. Observations made in one country or even in one region may not be appropriate to another country or to another region within the same country. These patterns change with time.
Hypertonic dehydration used to be common in the United States, as mentioned earlier. A high incidence of hyponatraemia and hypokalaemia has been reported from many places including the West Indies, Africa and among Australian aborigines. Kingston (1973) found that more than 80% of infants in Liberia with gastroenteritis had hypotonic dehydration. He has emphasized the importance of recognition of hypokalaemia in these circumstances and its appropriate treatment. Kingston believes that continued intake of small amounts of breast milk at frequent intervals while diarrhoea continues is an important factor in the infants he described, as the sodium and potassium contents of breast milk are so low (Table 6.11 ). He considers that a different pattern of water and electrolyte disturbance is seen when gastroenteritis occurs in breast-fed infants, as distinct from formula fed children. Table 6.11 indicates the amounts of Na+ and K+ found in breast and cows' milk but the range for breast milk is very wide.
Table 6.11.
Mean values for Na+ and K+ (mEq 1−1)
| Na+ | K+ | |
|---|---|---|
| Breast milk | 6 | 13 |
| Cows' milk | 23 | 37 |
The amount of Na+ and K+ in breast milk is far less than the average stool electrolyte losses (see Table 6.13 ) and hence the net loss of Na+ and K+ is relatively in excess of the net loss of water in breast-fed infants with gastroenteritis. This is a most unusual finding in Western countries where gastroenteritis is so uncommon in breast-fed infants. These considerations have important implications in the management of gastroenteritis, which will be discussed later.
Table 6.13.
Electrolyte composition of stool in childhood gastroenteritis
| Aetiology |
Stool electrolytes (mmol l−1) |
|||
|---|---|---|---|---|
| Sodium | Potassium | Chloride | Bicarbonate | |
| Cholera | 101* | 27 | 92 | 32 |
| 88† | 30 | 86 | 32 | |
| Rotavirus | 37† | 38 | 22 | 6 |
| ETEC | 53† | 37 | 24 | 18 |
| Non-cholera diarrhoea | 56* | 25 | 55 | 14 |
ETEC: enterotoxigenic E. coli.
Mahalanabis, et al. (1970).
Molla et al. (1981).
Acidosis
Various theories have been put forward to explain the aetiology of the metabolic acidosis that occurs in infants with severe gastroenteritis accompanying clinical dehydration. At present it seems that excessive intestinal losses of bicarbonate has been shown to be a major cause of their acidosis. Blood gas estimation together with the measurement of blood pH are useful investigations in a child who is severely dehydrated.
Acute renal failure
When this occurs as a complication of gastroenteritis, it may be due to oligaemia, producing reversible failure of renal function. This responds well to intravenous fluids. Much less commonly, it may be due to acute renal tubular necrosis. The prognosis of such a complication is often not good but it may respond to appropriate management.
Management
The successful therapy of gastroenteritis in children relies upon the maintenance or restoration of adequate hydration and electrolyte balance. In most children this may be achieved by manipulating the diet but when this fails and significant dehydration occurs intravenous fluids are required. Wheatley (1968) found that nine out of ten children could be managed successfully at home, and only one required hospital admission.
Diet therapy
The principles of this management are to stop all solids and to replace the child's milk feeds with a glucose electrolyte solution in infants or clear fluids in older children given hourly or two-hourly for 24 hours. Usually, vomiting and diarrhoea stop under this regimen. If this does not happen, the child is often admitted to hospital for observation because the child has a severe form of gastroenteritis and may require intravenous fluids, or there is some other malady, or possibly his mother has not been giving him the oral regimen as recommended.
There is no general agreement about the precise formulation for a glucose electrolyte solution but it is clear that whatever is given the aim should be to provide a total fluid intake greater than about 20% of the child's usual fluid requirements so that the input of fluid will keep pace with the fluid loss.
Table 6.12 indicates the theoretical fluid requirements in ml kg−1 at various ages throughout childhood.
Table 6.12.
Fluid requirements in childhood
| Age | Daily fluid requirements (ml kg−1) |
|---|---|
| 1st day of life | 60 |
| 2nd day of life | 90 |
| 3rd day of life | 120 |
| Up to 9 months | 120–150 |
| 12 months | 90–100 |
| 2 years | 80–90 |
| 4 years | 70–80 |
| 8 years | 60–70 |
| 12 years | 50–60 |
Dietary management: oral rehydration therapy
A wide range of oral replacement fluids has been recommended for children with gastroenteritis. Table 6.13 shows a comparison of the electrolyte content of diarrhoeal fluid in childhood gastroenteritis.
Weil has laid down certain guiding principles in relation to the oral feeding referred to earlier, but it is apparent from Table 6.14 that very widely ranging solutions in relation to electrolyte content are used. Glucose has been recommended for many years, e.g. by Darrow and his colleagues in 1949, and evidence has now been produced to show that there are good reasons for using it. There is coupled absorption and transport for glucose and sodium in the small intestinal mucosa and the absorption of sodium and water is consequently greatly stimulated by luminal glucose. Hirschhorn and his colleagues (1973) and others have found that glucose-electrolyte mixtures given by mouth or by intubation greatly diminish the intestinal loss of fluids and electrolytes in children with cholera.
Table 6.14.
Composition of WHO and other glucose electrolyte solutions (mmol I−1) in the UK
| WHO- | WHO-CITRATE | GEM | DIORALYTE | REHIDRAT | DEXTROLYTE | |
|---|---|---|---|---|---|---|
| Bicarbonate | 30 | 18 | 20 | |||
| Citrate | 10 | 30 | 9 | |||
| Lactate | 8 | |||||
| Sodium | 90 | 90 | 24 | 35 | 50 | 35 |
| Chloride | 80 | 80 | 24 | 37 | 50 | 30 |
| Potassium | 20 | 20 | 28 | 20 | 20 | 13 |
| Calcium | 4 | |||||
| Magnesium | 4 | |||||
| Glucose | 111 | 111 | 277 | 202 | 91 | 200 |
| Sucrose | 94 | |||||
| Fructose | 2 | |||||
| Osmolality (mosmol kg−1) | 331 | 351 | 310 | 336 | 297 |
This type of therapy has been extended to infants with acute diarrhoea due to other causes, with great success. It has led to a great fall in mortality from acute diarrhoea. It is known as oral rehydration therapy or ORT. In the developing world the WHO or UNICEF solution (WHO-ORS) is most widely used. ORT is discussed in more detail in Appendix II. Its sodium and potassium composition is indicated in Table 6.14 together with other solutions.
The sodium content of this solution is within the range of the sodium content found in diarrhoeal fluid but it is higher in electrolyte content than the other solutions. Unless large volumes are taken or free water as well there is a risk of hypernatraemia with this solution, except in communities where hyponatraemia is common, e.g. communities where there is a high incidence of protein-calorie malnutrition. Also, if diarrhoea ceases immediately, too much sodium can be given, especially in small infants. However, in view of the declining importance of hypernatraemic dehydration in developed countries, owing to widespread use of low solute milks and better education, this risk may not now be so great.
Before the advent of the concept of oral rehydration therapy, oral half-strength Darrow's solution was widely used in developing countries and continues to be used successfully in some countries. Electrosol tablets when dissolved produce an adequate electrolyte solution for oral use, but as tablets they do carry the risk of being given in excess dosage. Half-strength saline contains no potassium and significant potassium loss does occur in gastroenteritis, but in emergencies this solution flavoured with a little orange juice may be very useful until other solutions can be obtained. There are risks of giving soft drinks in a casual manner as their electrolyte content and osmolality varies greatly (Table 6.15 ). A glucose-electrolyte mixture (GEM) was used at Queen Elizabeth Hospital for children from 1952 to 1980. Its composition was based on one devised by the Medical Research Council in 1952. It was similar to the first solution used by Darrow.
Table 6.15.
Analysis of popular soft drinks
| Brand | pH | Osmolality (mosmol kg−1) |
Electroylytes (mmol l−1) |
|
|---|---|---|---|---|
| Sodium | Potassium | |||
| Coco-Cola | 2.8 | 469 | 3.0 | 0.1 |
| Pepsi-Cola | 2.7 | 576 | 1.0 | 0.1 |
| Seven-Up | 3.5 | 388 | 4.0 | 0.0 |
| Lucozade | 3.0 | 710 | 18.0 | 0.5 |
| Orange juice | 4.0 | 587 | 1.0 | 46.0 |
| Apple juice | 3.6 | 694 | 0.0 | 27.4 |
| Ribena* diluted 1:3 | 3.0 | 1180 | 4.0 | 8.0 |
| Ribena diluted 1:4 | 3.3 | 862 | 3.0 | 6.0 |
| Ribena diluted 1:5 | 3.4 | 561 | 2.0 | 4.7 |
Blackcurrant cordial.
Reproduced from Head et al. (1983).
© 1988
Its composition is indicated in Table 6.14. Its use was stopped for economic reasons in 1980 when a commercially available solution Dioralyte (Armour Pharmaceuticals) was introduced. The glucose electrolyte solution (GEM) used at Queen Elizabeth Hospital contained some calcium and magnesium because it had been noted that some infants with acute diarrhoea had low plasma levels of these electrolytes. In relation to more recent formulations it is interesting to observe the relatively high glucose composition of 50 g glucose/litre, the low sodium level and the use of citrate. This solution was used very successfully for almost 30 years.
However, it was not used for children who were 5% dehydrated or more who were given intravenous fluids. This mixture was a ready-made solution and once opened it had a short ‘shelf life’ because of the risk of bacterial contamination. Hence inpatients only were given this solution at Queen Elizabeth Hospital whereas outpatients were given an oral sucrose-electrolyte solution known as out-patient electrolyte solution (OPEM). The basic electrolyte solution was supplied in a concentrated form and diluted five times with water at home. Then sucrose was added at home. It was thus hoped to avoid bacterial contamination.
In view of the theoretical advantages of glucose in cholera toxin induced diarrhoea and the experience of its use in diarrhoeal states not due to cholera, by Hirschorn and colleagues (1973), as well as the theoretical drawback of using a surcrose solution because of the documented low sucrase levels found in the small intestinal mucosa in acute gastroenteritis (Barnes and Townley, 1973), on two occasions a clinical comparison of the use of glucose and sucrose additions to a basic electrolyte mixture in the outpatient management of acute gastroenteritis in children was undertaken (Rahilly et al., 1976; Hutchins et al., 1979).
In this latter study it was found that when the osmolality of the solutions as made up by the mothers was assessed it was remarkably variable. This could be detrimental leading to osmotic diarrhoea if hyperosmolar solutions were used. In fact Ironside (1973) had cautioned against the unrestricted use of glucose in home-made solutions because of the risk of making up a hypertonic solution and so aggravating the clinical situation. To investigate this further a study was undertaken of the variations in osmolality of samples of oral solutions as provided by parents (Hutchins et al., 1980) (Figure. 6.13 ). The following solutions were compared, first parents diluted a concentrated outpatient electrolyte mixture (glucose OPEM) and then added glucose or sucrose (sucrose OPEM), next parents added sucrose at home to an already diluted electrolyte mixture, then this solution as prepared by pharmacy staff was studied and finally a sachet of Dioralyte (based upon sodium chloride and dextrose compound powder BPC) to which water was added at home was studied.
Figure 6.13.
Osmolalities of solutions in study of Hutchins et al. (1980).
All mixtures except those prepared by the pharmaceutical staff were often made up with marked inaccuracy. It was only with solutions made up with glucose that dangerously high levels of osmolality were reached. For sucrose even when inaccurately made up the levels of osmolality barely exceeded the correct value for the glucose solutions.
These two studies at the Queen Elizabeth Hospital were carried out on outpatients attending casualty during the winter months, 2 years apart. Both showed that, in those given GEM (27% and 32% respectively in the two studies) more children experienced failure with out-patient management and required admission compared with those given OPEM (10% and 18%). As social reasons often contributed to failure in outpatient management, these studies can only be cited as evidence that a sucrose electrolyte solution appeared to be at least as good as glucose in the outpatient management of acute gastroenteritis in a developed community.
The second study (Hutchins et al., 1978) did, however, make it clear that it was easier to make an error when the solution was made up with glucose and could lead to unacceptably high levels of osmolality in some instances. Such high levels could contribute to outpatient management failure. The author commends the use of a sucrose electrolyte solution with its lower osmolality, ready availability, and economic advantages, both in developed and also in developing countries, from which encouraging results have been reported, from Indonesia (Moenginah et al., 1975) and also from Calcutta (Chatterjee et al., 1977). A plea was made for the use of sucrose on the basis of these observations but commercial formulations have largely continued to use glucose.
One other issue the study of Hutchins et al. addressed was the concentration of sodium. However as all solutions were low in sodium content none were associated with dangerously high levels. When high content sodium sachets, such as the WHO formulation are used, this is a risk (Cutting, 1979).
Since 1980 commercial formulations have been used at Queen Elizabeth Hospital. Initially a sachet preparation (Dioralyte) was used. This has the advantages of compactness and convenience over an already made up solution but as seen in Figure. 6.13 it does carry the occasional risk of being made up inaccurately and so producing a dangerously high osmolality. Therefore a study was made of a ready-to-use glucose electrolyte solution (Dextrolyte, Cow & Gate) in the outpatient management of infants with acute gastroenteritis (Trounce et al., 1985). This showed this solution was readily accepted by parents and children. Twenty-two samples of the mixture as actually given to the baby were brought for analysis. The osmolality ranged from 291 to 308 mosm l−1 with a mean of 298 mosm l−1. Thus in no case was there a dangerously high level. A disadvantage of ready to feed solutions is the risk of bacterial contamination once the bottle is opened. At present such solutions are presented in 100 ml bottles and parents are advised to discard the unused portion. If such solutions are refrigerated even when the bottle is opened and used again the risk of bacterial contamination is minimal (Santosham et al., 1982). These workers showed that if the solution is stored at 4°C after the introduction of 1000 colonies of E. coli it will remain sterile 24 hours later, whereas if it is stored at room temperature the E. coli will, not surprisingly, grow freely.
Experimental animal work from Hamilton et al. (1976) provides a theoretical basis for the view that there is no specific benefit in using a glucose electrolyte solution when there is viral gastroenteritis. Using a transmissable gastroenteritis virus (TGE) to study gastroenteritis in pigs, they have been able to study glucose-stimulated sodium transport which in fact falls to its lowest level at the peak of the diarrhoea. Thus it seems probable that in viral gastroenteritis, carbohydrate electrolyte solutions merely provide calories and are unlikely to have a specific effect on sodium absorption. These studies do show the virtue of a lower glucose level as currently in WHO-ORS solution (see Appendix II).
Dietary management: bottle-fed infants
In the United Kingdom for many years it has been the practice after 24 hours of a glucose electrolyte mixture (GEM) to gradually reintroduce the infant's usual feeding formula or cows' milk back into his diet by a system known as regrading. The speed of this process of regrading will depend upon the individual infant's circumstances; in particular, the severity of his vomiting and diarrhoea during the acute episode and the persistence or otherwise of such symptoms. Regrading is usually done in quarter-strength increments, i.e. starting with quarter-strength dilution of the milk feeding with the glucose electrolyte mixture moving up to full strength in quarters (Table 6.16 ) at intervals of 24 hours. This is a slow regrade. When the increments occur at 6 or 12 hours this is known as a fast regrade. The rationale for this conventional approach is based upon the following points:
-
•
The perceived need to provide a short period of relative bowel rest by giving dilute feed of increasing strength following the acute onset at a time when the small intestinal mucosa is known to be damaged (at least in the case of viral diarrhoea, see page 223) and is then recovering.
-
•
To prevent a significant return of symptoms, i.e. relapse, by rapidly returning to a lactose containing feed which might result in osmotic diarrhoea in view of the state of temporary lactase deficiency related to the small intestinal mucosal damage present at this time or due to malabsorption of some other nutrient by the damaged mucosa.
-
•
To prevent food (i.e. cows' milk) sensitization by reducing antigen load at a time when the small intestinal mucosa is abnormal and there is evidence of increased antigen uptake (Gruskay and Cooke, 1955) and increased permeability to sugar (Ford et al., 1985). Both factors predispose to increased antigen entry to the mucosa and so to sensitization.
Table 6.16.
Table of suggested regrading of feeds
| Regrading by quarters ml/kg corrected weight | ||
|---|---|---|
| Day 1 | GEM* | 150 |
| Day 2 | GEM | 115 |
| Milk feeding | 35 | |
| Day 3 | GEM | 75 |
| Milk feeding | 75 | |
| Day 4 | GEM | 35 |
| Milk feeding | 115 | |
| Day 5 | GEM | — |
| Milk feeding | 150 |
GEM = Glucose-electrolyte mixture.
Once the infant is on full-strength formula again, solids may be reintroduced to his diet. It is important for the mother and indeed for the doctor to remember that some looseness of stools may persist for several days after gastroenteritis and that it may take time for the stools to return to normal.
This established approach has been challenged from two directions. First, by studies which question the necessity for such regrading; and second, by those who consider that food intolerance is so common a sequel to acute gastroenteritis that a lactose-free hypo-allergenic feed should be given at this time.
Both these challenges need to be considered. However, it must be made clear that the concept of regrading has arisen in developed communities. Here nutritional reserves are generally sufficient to offset the detrimental effects of actue episodes of diarrhoea as the children are usually previously well nourished. Furthermore the type of infant feeding has changed in recent years to adapted formulae that are less sensitizing than doorstep milk.
First considering the need for regrading, as long ago as 1948 Chung performed detailed studies on food absorption in infants with acute gastroenteritis. He showed that duration of diarrhoea was not prolonged in infants continuing to receive full strength feeds compared with those placed on a starvation regime. However his views were not generally accepted in practice. More recently, others have challenged the need to regrade (Rees et al., 1979; Dugdale et al., 1982; McDowell and Evans Jones, 1985; Haque et al., 1983). There have been warnings from the developing world that dilute feeds may lead to malnutrition but, as stated earlier, only developed countries are being discussed here.
Placzek and Walker-Smith (1984) at Queen Elizabeth Hospital studied this problem in 48 children less than 18 months of age admitted with acute gastroenteritis (see Table 6.20 for clinical features). Complicated progress, i.e. relapse, occurred in 7 (30%) where no regrade was used and only 1 (4%) where a regrade was used. Relapse was most often due to lactose intolerance but was due to severe vomiting in two. However, the complications entirely occurred in children under the age of 9 months. This risk of relapse was confined to children under the age of 9 months. Thus it was considered that in children older than 9 months gradual reintroduction of milk was not necessary. However, for those aged under 9 months there was a significantly greater risk of relapse due mainly to temporary lactose intolerance associated with rotavirus infection. A similar result was obtained in Chester (McDowell and Evans-Jones, 1985) although they interpreted their results differently (Walker-Smith, 1986).
Table 6.20.
Recent outbreaks of salmonella poisoning: distribution (%) of signs and symptoms
| Symptoms and signs | Non-typhoid salmonellae (n = 46) | S.typhi(n = 2) | S. paratyphi (n = 2) |
|---|---|---|---|
| Diarrhoea | 41 | 2 | 1 |
| Vomiting | 28 | 2 | 1 |
| Fever (>38°C) | 25 | 2 | 1 |
| Blood in stool | 12 | 0 | 1 |
| Abdominal pain | 12 | 0 | 1 |
| Cough | 8 | 2 | 1 |
| Swinging fever | 8 | 2 | 0 |
| >5% dehydrated | 2 | 0 | 0 |
| Rose spots | 0 | 1 | 0 |
The numbers in this study were still relatively small and only concerned inpatients. Therefore another study was undertaken at Queen Elizabeth Hospital of 68 babies admitted with acute gastroenteritis under 6 months of age (Armistead et al., 1987); 39 were admitted to Hospital and 29 attended a daily outpatient clinic. Three groups were studied: a control group (I) regraded in the conventional way; group II, no regrades; group III, regraded onto the hypoallergenic whey hydrolysate formula Alfaré (Table 6.17 ). There was no significant difference in relapses between the three groups.
Table 6.17.
Complications in 68 infants after gastroenteritis
| Group I (n = 22) | Group II (n = 22) | Group III (n = 24) | |
|---|---|---|---|
| No complications | 17 | 14 | 14 |
| Relapse: | 4 | 5 | 3 |
| Stool pathogens on relapse | 2 | 4 | 2 |
| Cows' milk protein intolerance | 1 | 1 | 1 |
| Refusal of feed | 0 | 0 | 6 |
| Exclusion for other medical reasons | 0 | 0 | 1 |
| Lost to follow-up | 1 | 3 | 0 |
However, no child developed lactose intolerance. Relapses were due to a return of diarrhoea without excess stool reducing substances. This study differed from the earlier study as there were no cases of previous failure to thrive and there were fewer severely dehydrated.
In communities where malnutrition is common it is important that there should not be any prolonged reduction of caloric intake following gastroenteritis and it is vital that a starvation diet for a long interval should be avoided. Except during acute water and electrolyte disturbance the diet of malnourished children should not be unduly restricted even though increased feedings may accentuate diarrhoea. Provided this does not lead to dehydration and further electrolyte disturbances in these children, it is important to continue with an oral feed containing an adequate amount of calories. Such malnourished infants tolerate further caloric restriction very poorly and diarrhoea may even become worse with the untoward consequences. This is a quite different situation from that of the previously well-nourished child who develops gastroenteritis.
Intravenous therapy
Intravenous therapy for dehydration in man was first used by a Scottish general practitioner, Dr Thomas Latta, in 1832, with some success, but it was a long time before this treatment became accepted. Gamble (1953) reviewed the subsequent history of this technique, and it was indeed notably Gamble, and also Darrow and colleagues, who in the 1940s and 1950s put safe intravenous therapy in childhood on the therapeutic map. The subject has been well reviewed by Weil (1969) and Finberg (1970).
Fortunately, in the developed world, despite the continuing importance of acute gastroenteritis, the numbers of children with severe dehydration requiring intravenous fluids is falling. In 1973, at the Queen Elizabeth Hospital, only 60 from the 353 children admitted with acute gastroenteritis required intravenous fluids i.e. 16% (Gribbin et al., 1976). The most recently published figure of 1.4% from the UK (Cutting et al., 1986) is even lower. Nevertheless, the situation still may be urgent. In the developing world severely dehydrated children requiring intravenous fluids continue to be a common problem.
There are three important phases in intravenous therapy; namely resuscitation, rehydration and maintenance. These will be outlined first of all for children with isotonic dehydration.
Resuscitation
If a child is shocked (i.e. he is pulseless or has a low blood pressure and peripheral cyanosis) he needs a rapid infusion of fluids as a matter of urgency. He may require up to 15-20 ml fluid per kilogram of body weight to be administered over 10-15 minutes, to rapidly restore his state of acute oligaemia. More often a child who needs resuscitation is not so severely ill and may just have some peripheral cyanosis and a history of oliguria. He should then have an initial infusion of between 40 and 80 ml/kg actual body weight given over 4 hours. Such an infusion is considered adequate if at least 10 ml/kg actual body weight of urine is passed within the first 4 hours of the infusion. If inadequate urine is obtained the infusion should be continued and the clinical situation reviewed. If anuria persists then the child should be managed as for renal failure. This will be discussed further under management of complications. Table 6.18 is a guide to the amount of fluid that should be given during this resuscitation phase.
Table 6.18.
Volume to be given over 4 hours
| 5% dehydration | 40 ml kg−1 |
| 5–7% dehydration | 60 ml kg−1 |
| 10% dehydration | 80 ml kg−1 |
The infusion fluids given vary considerably from unit to unit but 0.45 per cent sodium chloride in 5 per cent dextrose, half-strength Darrow's solution, fresh frozen plasma, a stable plasma protein solution, or an albumin solution may all be used (Table 6.19 ).
Table 6.19.
Composition of intravenous fluids (mEq l−1)
| Sodium | Potassium | Chloride | Calcium | Lactate | |
|---|---|---|---|---|---|
| Isotonic or physiological saline | 154 | — | 154 | — | — |
| Half isotonic (dextrose 5%, sodium chloride 0.45%) | 77 | — | 77 | — | — |
| Quarter isotonic (dextrose 3.75%, sodium chloride 0.225%) | 38 | 38 | — | — | |
| Fifth isotonic (dextrose 4%, sodium chloride 0.18%) | 30 | — | 30 | — | — |
| Hartmann's solution | 131 | 5 | 112 | 4 | 28 |
| Half-strength Darrow's solution | 60 | 18 | 52 | — | 25 |
Once the child's circulation has been restored and he is passing urine, care should be taken not to give him too much fluid. Constant reassessment, clinically, is necessary.
Rehydration
When the infant is not oliguric and there is no peripheral circulatory failure, an initial infusion is not required. He should be rehydrated according to a calculation based on a clinical estimate of his percentage dehydration. When the infant who requires resuscitation has had an initial infusion and he is passing urine he should then be reassessed and future fluid requirements calculated according to his current estimated percentage dehydration. Caution should be used in rehydration, for too rapid rehydration may lead to central nervous system disturbances with convulsions. An infant who is estimated to be 5% dry is rehydrated over 24 hours. The calculated amount of fluid for rehydration is given as half-strength isotonic or 0.45% saline with dextrose, either 2.5% or 5% and this added to his normal daily maintenance fluid requirement.
The volume of fluid given for rehydration in the first 24 hours should not normally exceed that calculated for a 5% deficit. When greater deficits are present the aim should be to rehydrate the child over 48 hours or more.
Example: child weighing 7.8 kg estimated to be 5% dehydrated
Amount of fluid to be given:
Intravenous therapy in relation to the aetiology of gastroenteritis—
In general, children require intravenous fluids when they are severely dehydrated regardless of aetiology. Relative specifically to rotavirus gastroenteritis, Hamilton et al. (1976) think that speedier cessation of diarrhoea may occur when intravenous fluids are used regardless of state of hydration. Certainly, Torres-Pinedo, Lavastida and Rivera (1966) have shown a sudden fall in stool electrolytes when milk is withdrawn from the diet of infants with gastroenteritis. There is a firm clinical impression that when intravenous fluids are given to these children with nil by mouth for a short period, recovery is rapid and uneventful. Nevertheless, it would not now be correct in most cases to use intravenous fluids unless the infant was at least 5% or more dehydrated.
Maintenance fluids
The usual daily maintenance fluid requirement ranges from 150 ml/kg body weight for an infant a few weeks old to 50–60 ml kg−1 for a child aged 12 years (see Table 6.14) and it should be given as 0.225% of 0.18% saline with glucose.
Daily sodium requirements are of the order of 2-3 mmol/kg body weight. Maintenance potassium requirements approximate 3 mmol/kg body weight. Administration of potassium should begin as soon as urinary output is satisfactory, care being taken not to infuse a concentration of potassium greater than 40 mmol 1−1 except in a severely malnourished child. NB Hartmann's and Darrow's solutions both contain potassium but in practice this usually does not appear to be a problem when these solutions are used for resuscitation. Their continued use in a child with anuria should, however, be carefully reviewed.
Investigations
Serum electrolytes, blood urea and total serum protein levels should be estimated as a matter of urgency in all children who are shocked, and within 24 hours in all children who are given intravenous fluid, for evidence of any electrolyte level disturbances, especially hypernatraemia, and as a confirmation of the state of dehydration. The levels of blood urea and total protein are sensitive indices of dehydration. Other useful investigations include haemoglobin and blood gas estimation when signs of acidosis are present.
Management of complications of fluid, electrolyte and acid-base balance
Hypernatraemic dehydration
The management of hypernatraemic dehydration is more difficult than that of isotonic dehydration and there is still controversy as to the best method.
The principal hazard of managing infants with hypernatraemic dehydration is the risk of water intoxication, with cerebral oedema. This may occur when rehydration is too rapid and may manifest as convulsions due to cerebral oedema.
Finberg (1973) has reviewed the best therapy for this condition. Firstly, when circulatory failure is present in these infants, rapid correction of the failure is the aim, as with isotonic dehydration. He recommends the use of plasma or an albumin solution. Secondly, when there are no signs of circulatory failure but the child is anuric he recommends an initial infusion of a solution containing sodium, 75-80 mmol 1−1 in 5% glucose, but no added potassium until urine is passed. Thirdly, as the next phase in management in these children, or the initial phase in those infants without either circulatory failure or anuria, he recommends slow rehydration over 48 hours with a relatively dilute solution and a sodium concentration of 25–40 mmol l−1 to which potassium is added.
There are risks from giving the higher sodium solution too rapidly or for too long, and also from giving potassium too soon. Provided there is no circulatory failure and no anuria the guiding principle should be to rehydrate the child slowly to allow physiological adjustment to occur. The aim is to lower serum sodium slowly as well as slowly to restore hydration; rapid reduction of serum sodium by rapid rehydration with a dilute solution may cause cerebral oedema with convulsions.
Hyponatraemic dehydration with hypokalaemia
Despite the low sodium level when hyponatraemia is present in malnourished children, Garrow Smith and Ward (1968) have shown that total body sodium is usually raised, hence 0.18% saline (1/5 isotonic saline) is recommended for rehydration as well as maintenance. In the previously well-nourished child, the regimen described above for isotonic dehydration is appropriate, except when levels below 110 mmol 1−1 occur, when extra sodium may be required. When there is severe hypokalaemia (serum K+ less than 1.5 mmol 1−1) in a severely malnourished child, large amounts of K+ may need to be given, 80 or 100 mmol l−1. This is because Kunin, Surawic and Sims (1962) have shown that infusion of moderate concentrations of K+ together with glucose leads to a further fall of serum K+ due to re-entry of K+ into the depleted intracellular space.
These differences in the management of dehydration which depend upon the type of dehydration present and the presence of pre-existing malnutrition, emphasize the importance of adjusting the type of management to the pattern of disease in a particular community. There is no universal way of managing all children with dehydration.
Acidosis
Partial correction of the acidosis with intravenous bicarbonate may be of great value in desperately ill infants, the aim being to correct half the calculated base deficit. Care should be taken not to infuse the bicarbonate too rapidly into the child's veins as there is then a risk of cardiac arrest. It should be added to the other intravenous fluids. In less severely ill infants, correction of the dehydration alone will restore acid-base balance and bicarbonate therapy is unnecessary.
Acute renal failure
Once acute oliguria, i.e. a urine output of less than 10 ml kg−1, has been present for more than 4 hours after the intravenous fluids have been started, Wharton (1968) has recommended considering the use of frusemide intravenously. Frusemide, when given intravenously, may prevent minimal renal shut-down. A dose of 1 mg kg−1 may be given provided the blood urea is not more than 50 mmol 1−1 as it is in about 1% of children with gastroenteritis. If this fails renal dialysis would then have to be considered.
Drug therapy
Anti-diarrhoeal drugs and anti-emetics are not recommended for most infants and children with gastroenteritis, although in the older child, where vomiting is the dominant symptom, intramuscular antiemetics, e.g. prochlorperazine (Stemetil) given in an appropriate dosage may very occasionally be useful. Prochlorperazine suppositories should “never be given as their absorption is unpredictable and the therapy may be complicated by dystonic reactions. The incidence of such side effects is much higher in children than in adults. Diphenoxylate hydrochloride (Lomotil), morphine derivatives and preparations containing kaolin and pectin appear to have little value in the small child with gastroenteritis, although they may afford symptomatic relief in the older child or adolescent.
Yet, despite lack of convincing evidence for their effectiveness, anti-diarrhoeal drugs often continue in practice to be prescribed for infants and young children. The best evidence for their lack of effectiveness is provided by the study of 80 children, aged 3–11 years, with acute diarrhoea in Guatemal (Portnoy et al., 1976). They found that kaolin and pectin preparations and diphenoxylate hydrochloride were no different from placebo in their effect on stool frequency, stool water content or stool weight, although kaolin-pectin concentrate did produce fewer liquid stools. However, this effect is purely aesthetic and of no value to the child. The use of these drugs in younger children should be resisted.
In the case of loperamide there was some evidence of advantage in its use when used as an adjunct to oral rehydration in the management of acute diarrhoea in well children (Evans et al., 1984). Whilst loperamide in dose of 0.8 mmol kg−1 daily speeded recovery by 24 hours, this is only a marginal advantage. The author does not believe that there is any advantage in using loperamide for the routine management of acute diarrhoea due to gastroenteritis. In chronic diarrhoea and in acute exacerbations of chronic diarrhoea there may be a place in management. This is discussed in Chapter. 13.
Antibiotics have little place in the management of children with gastroenteritis. This is because:
-
•
In most children, no bacterial pathogen is isolated from the stools.
-
•
Antibiotics may sometimes prolong the carrier state, for example in salmonella infections (Dixon, 1965).
-
•
There is little evidence that antibiotics influence the natural history of the disease even when bacterial pathogens are present.
-
•
There is conflicting evidence as to whether antibiotics eliminate these pathogenic organisms from the gut.
-
•
Consequently, it is uncertain whether they can prevent the spread of infection from one child to another.
Not only is antibiotic therapy ill conceived per se in the vast majority of infants with acute diarrhoea but such use may be an important contributing factor to the transfer of antibiotic resistance determinants from commensal bacteria to enteric pathogens. There is clear evidence that a high prevalence of antibiotic resistance in commensal bacteria does occur in children in developing communities where antibiotics are inappropriately prescribed (Shears et al., 1987).
Antibiotics are indicated when blood stream infection occurs in children with salmonella infections, but obviously antibiotics should also be given if there is an intercurrent indication, e.g. coexistent otitis media. Their place in the management of enteropathogenic E. coli infections and shigellosis is controversial. Neomycin has been claimed by some authors to be effective in eliminating these organisms from the stools; intramuscular ampicillin therapy has been claimed by others to be more effective in shigellosis. There are also reports of neomycin-resistant strains of enteropathogenic E. coli, colistin then being recommended as a substitute. There is no general agreement on the role of antibiotics in these infections and some authors are of the opinion that there is no convincing evidence that antibiotics do the least good in such children. Ironside (1973) has commented that their indiscriminate use may lead to the transfer of antibiotic resistance to other coliforms and that their use is a form of environmental pollution.
Specific syndromes
Viral gastroenteritis syndromes
The clinical associations described here are based largely upon experience of gastroenteritis at Queen Elizabeth Hospital since 1973. The hospital is situated in an area of socioeconomic deprivation with a large number of children of immigrants and a high incidence of gastroenteritis, with between 400 and 500 admitted most years and many more children seen as outpatients. The clinical associations which are described concern observations made on in-patients. However, Weindling, Walker-Smith and Bird (1980), reported the results of an investigation of 58 infants with gastroenteritis treated as outpatients at Queen Elizabeth Hospital and 30 controls with diarrhoea. The study was conducted in the autumn of 1977.
Rotavirus was found in 7% compared with 12% in-patients at the same time. Adenovirus was found in 3.5% of outpatients and 4% of in-patients. Neither virus was identified in controls.
Rotavirus
Bishop and her cplleagues (1973) used electron microscopy to demonstrate for the first time the presence of rotavirus particles in the enterocytes of six small intestinal biopsies from children with acute gastroenteritis.
Since then, rotavirus gastroenteritis has come to be recognized as the most important cause of acute diarrhoea in children between 6 months and 2 years of age in industrialized countries. In developing communities only enterotoxigenic E. coli rivals rotavirus in importance in most reports, although studies from such communities do vary in their assessment of the relative importance of rotavirus in the causation of acute diarrhoea. Diagnosis is usually based on electron microscopy of negatively stained stools as first reported by Flewett et al. (1973) or by enzyme-linked immunosorbent assay (ELISA) analysis of stools. This latter technique is now widely used diagnostically.
Virus morphology
Rotaviruses belong to the family of viruses known as Reoviridae. They are classified as a genus within this family. Rotavirus particles are made up of two concentric icosahedral protein capsids up to 60–70 nm in diameter, enclosing 11 segments of genomic double-stranded RNA. Each segment of RNA represents one gene which encodes one virus-specific protein.
Rotavirus has been found to infect a wide range of animals as well as man, e.g. calves.
The virus occurs in two forms. One is about 70 nm in diameter with a double-shelled capsid structure having a sharply defined circular outline with the appearance of a rim of a wheel, hence the name, rota from the Latin for a wheel (Figure. 6.14 ). Then there are smaller (55 nm diameter) rougher particles which appear to be viruses that have lost their outer shell; these have been reported not to be infective.
Figure 6.14.
Rotaviruses in the enterocyte of a biopsy sample from a child with acute gastroenteritis.
(Reproduced from Bishop (1973) by kind permission of author and publishers.)
© 1988
Electron microscopy may reveal large numbers of virus particles packed together or there may be only scattered virus particles. Concentrations of more than 106 particles/ml are necessary for the agent to be seen with the electron microscope by routine negative staining techniques.
Varieties of rotavirus
Rotaviruses may be typed by immunological or biochemical methods. Flewett et al. (1974) presented evidence from immune electron microscopy that type-specific antigens of rotaviruses were located on the outer capsid shell, whereas the group antigen for rotavirus distinguishing this virus from other viruses was located in the inner capsid of the virus particle. Since that time using a variety of immunological methods, rotaviruses can now be classified into groups, subgroups and serotypes.
Group antigen initially appeared to be common to all rotaviruses but it is now known that very occasionally some rotaviruses cannot be detected by the conventional way to detect the group antigen. In fact there are now five group antigens recognized (A-E). Most rotaviruses belong to Group A. There are two main subgroup antigens (1 and 2) but non-1 and non-2 viruses are found. There are now seven serotype antigens recognized. Before rotaviruses could be cultured these were thought to relate directly to antibody serotypes in the patient's serum but this is not so. All this progress has been related to neutralization assays and cell culture facility. This is very time consuming. The use of monoclonal antibodies would be much simpler. Shaw et al. (1985) have described monoclonal antibodies against two serotypically distinct rotavirus strains: Wa, a serotype 1 virus of human origin and a rhesus rotavirus, a simian serotype 3 virus.
However, monoclonals are not yet available for all serotypes and furthermore the situation is complex as the same antigens may be shared by different serotypes. This may make the practical application of monoclonals difficult.
Biochemical analysis is based on polyacrylamide gel electrophoresis of rotavirus RNA to demonstrate specific electropherotypes. In fact considerable genetic variability among human rotaviruses has been observed based upon the diversity of the electrophoretic patterns shown by viral RNAs from rotaviruses recovered from the stools of ill children. Flores et al. (1985) have studied stools from children with rotavirus gastroenteritis using this technique. They proposed two major rotavirus ‘families’ corresponding to human rotavirus prototypes Wa (subgroup 2, human serotype 1) and DS-1 (subgroup 1, human serotype 2). Most rotaviruses belong to these two families. This technique is a useful tool for epidemiological study and is discussed later.
Pathogenicity of rotavirus
Flewett (1976a) has pointed out that in contrast to a bacterium one can hardly hope to fulfil all Koch's postulates before accepting that a virus particle found in the stool is a pathogen. This difficulty relates to ethical reasons as well as difficulty in culturing a virus. He has listed some of the evidence that may reasonably be expected to establish the pathogenicity of a stool virus. Some of these are fulfilled for rotaviruses and include the following.
Particles found in material from patients with the disease but not from patients with other unrelated disease
This, in general, appears to be true for rotavirus.
The prevalence of a particle in the population coincides with the prevalence of the disease
Again, this appears in general to be true for rotavirus outside the neonatal period but with some exceptions. Davidson et al. (1975a) found no particles in controls but Rodriquez et al. (1977) found rotavirus in 8% of 76 control children. However, Champsaur et al. (1984) described three categories of stool rotavirus excretors. First, there were those who had rotavirus gastroenteritis; second, those with asymptomatic infection, i.e. rotavirus with serological response and no symptoms; third, rotavirus carriers. Burke, Gracey and Masters reported (1985) in response to Champsaur et al. (1984) that 121 (10.5%) of 1156 children with diarrhoea and 51 (8.6%) of 593 children without diarrhoea excreted rotavirus! These observations suggest rotavirus has variable pathogenicity. A comprehensive cohort study has been reported from rural Costa Rica (Simhon and Mata, 1985) who found a low pathogenicity for rotavirus. This was strikingly different from their earlier studies in Santa Maria Caque in Guatemala where there was a high pathogenicity (Mata et al., 1983). However, the overall incidence of diarrhoea was much less in Costa Rica. They believe that the more heavily contaminated environment and the presumably larger infectious dose the children receive in Guatemala, may be the critical factors in explaining this difference.
However, there is clearly a general pattern emerging of different pathogenicity for rotavirus in different environments and at different times of the year as well as at different ages. The fact that antibody to rotavirus is acquired in the majority of children in developed countries who have not been to hospital indicates that most often the disease does not require hospital admission and is a mild illness.
Presence of the particle corresponds with the duration of the disease
Studies to date in general support this for rotavirus.
Purified particles induce the disease in adult volunteers or experimental animals
Infection induced in an adult volunteer has been shown by Middleton et al. (1974) and it has been transmitted to an infant monkey (Lambeth and Mitchell, 1975).
In summary rotavirus has been identified by electron microscopy in the duodenal mucosa, duodenal juice and stools of children with acute gastroenteritis, but far less often in controls in most studies. Thus there appears to be enough evidence to indicate that rotavirus is a significant pathogen causing gastroenteritis although of varying pathogenicity.
Epidemiology
Rotavirus has been found both in sporadic cases and also in epidemic outbreaks of acute gastroenteritis in childhood. Its epidemiology has recently been reviewed (Cotterill and Walker-Smith, 1986). Reports of its association with diarrhoea have come from the developed world including Australia, Europe and North America but also from developing communities in Africa and Asia. Its worldwide distribution demonstrates that it is a major pathogen of international importance. Rota-virus is indeed the most important cause of acute gastroenteritis in children between 6 months and 2 years in developed communities and is only rivalled by enterotoxigenic E. coli in developing communities.
Rotavirus in children in developed world
Characteristically in temperate climates, rotavirus has been identified in the stools of children during the winter peak of gastroenteritis. A winter peak in the prevalence of non-bacterial gastroenteritis has been known for some time in developed countries both in the northern and southern hemispheres. As long ago as 1929, Zahorsky described winter vomiting disease and, in 1973, two winter epidemics of non-bacterial gastroenteritis were described in north-west London (Sinha and Tyrell, 1973). A study of monthly admissions to a gastroenteritis unit in Sydney from 1961 to 1972 showed the sudden appearance of a winter peak in hospital admissions in 1964, which persisted in subsequent years (see Figure. 6.12). An association between this winter peak and rotavirus in the stools was reported from Melbourne (Davidson et al., 1975b), Toronto (Hamilton et al., 1976), Washington (Kapikian et al., 1976), Birmingham (Flewett et al., 1973) and London (Figure. 6.7), i.e. in cold and temperate climates. The peak prevalence may approach 80% among infants and young children during the winter months in North America (Kapikian et al., 1976; Hamilton et al., 1976). In some reports, the virus is not found at all during the summer months but in others it falls to a 20% level or thereabouts.
Rotavirus in children in the developing world
There are now increasing reports of studies of children with rotavirus infection from the developing world.
Maiya et al. (1976) found rotavirus in 13 of 50 children in tropical southern India (26%), the virus being found only during the cooler months of the year.
A study from Indonesia has studied the relative importance of infectious agents in children requiring hospital admission for acute diarrhoea (Soenarto et al., 1983). Rotavirus infection accounted for 27% of the infants under 6 months old, 41% aged 7-24 months, and 25% of those aged over 24 months (Figure 6.15, Figure 6.16, Figure 6.17 ). It was the most frequent infection in each age group. Overall, rotavirus was identified in 38% of all hospitalized children. Viruses were identified in the stools by electron microscopy. Another hospital-based study from the same region, Malaysia, studied the prevalence of rotaviruses over a 7-month period using an ELISA technique (Yap, Sakil and Muthu, 1984). Children aged between 1 and 2 years had the highest infection rate. Overall rotavirus was found in 46.3% of the children studied.
Figure 6.15.
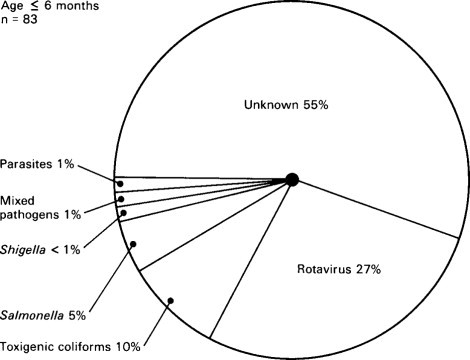
Relative importance of infectious agents in children aged under 6 months with diarrhoea requiring admission to hospital in Indonesia.
(Reproduced from Soenarto et al. (1983) by kind permission of authors and publishers.)
© 1988
Figure 6.17.
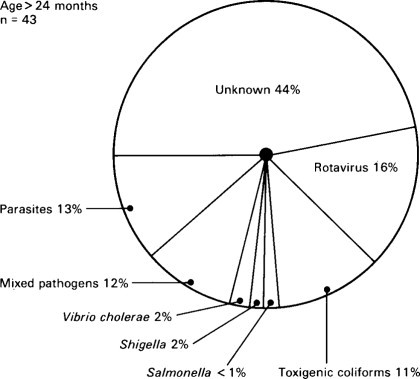
Relative importance of infectious agents in children aged over 24 months with diarrhoea requiring admission to hospital in Indonesia.
(Reproduced from Soenarto et al. (1983) by kind permission of authors and publishers.)
© 1988
In a report from Brazil of admissions with acute diarrhoea, rotavirus was found in 41.8% using enzyme immunoassay and electron microscopy for diagnosis (Coiro et al., 1983). Interestingly, this study showed that the disease most frequently occurred during the higher temperatures of a southern hemisphere summer in clear contrast to the winter peaks of temperate regions. Yet from another tropical country, namely Indonesia (Soenarto et al., 1983), it was reported that for most of the year, there was no seasonal variation except a decline during the change from dry to wet conditions in November and December. By contrast, in the Malaysian study, the number of cases of rotavirus increased from December to a maximum in March, having some relationship to an increase in rainfall at that time (Simhon et al., 1985).
So it is clear then that there may be a striking variation in the seasonal pattern of hospital-admitted cases of rotavirus in tropical countries, unlike the situation in temperate climates, where the winter peak is virtually a universal finding.
The most careful study of this issue in a tropical country comes from Guatemala. A cohort study of 45 Guatemalan Mayan Indian children, from birth to 3 years of age, has given a very clear account of the epidemiology of rotavirus in a classic study at Santa Maria Cauque (Mata et al., 1983). This constituted a close-knit, rural traditional society, living in poverty and in crowded unsanitary conditions. A retrospective ELISA analysis was made of frozen faecal specimens collected from 1966 to 1969.
This was related to growth and morbidity data. Rotavirus infections were uncommon in the first months of life in exclusively breast-fed infants. However, all children excreted rotavirus during the first 3 years of life. Some children had repeated episodes of rotavirus infection. Most infections lasted only 1 week but four lasted for 2 weeks and two lasted 5 weeks. Peaks in incidence coincided with the middle and late rainy season. Incidence rates were highest during the second year of life when administration of contaminated weaning foods was at its height. Whilst the incidence of diarrhoea was high in the cohort, rotavirus only accounted for 10% of the episodes of diarrhoea. In two-thirds of the cases, rotavirus was the only pathogen detected but other viruses and enterotoxigenic strains of E. coli were not sought.
Episodes of rotavirus-related diarrhoea were positively correlated with periods of growth abnormality. The episodes of infection were severe with frequent vomiting, dehydration and deterioration of nutritional status. Infection showed a high degree of communicability, affecting as many as one-half of all cohort children, especially during epidemic months. Thus it is clear that season may have a profound effect on the prevalence of this infection.
Pathology
Rotavirus particles have been identified by electron microscopy in the small intestinal mucosa of children with acute gastroenteritis in studies in Australia (Figure. 6.14) (Bishop et al., 1973; Holmes, Ruck and Bishop, 1975), and Japan (Suzuki and Konno, 1975) and by immunofluorescence in Canada (Middleton, Szymanshi and Abbott, 1974). With the exception of the Japanese reports, the particles have not been found in the lamina propria. Rotavirus was found in the enterocytes of 6 out of 9 children with acute gastroenteritis biopsied by Bishop et al. (1973), 1-5 days after the onset of symptoms. All biopsies revealed histological abnormality ranging from mild to severe but the intra-epithelial lymphocyte count was normal (Ferguson, McClure and Townley, 1976).
The virus particles in the enterocytes were found in distended cisternae of the endoplasmic reticulum and in the cisternae between the inner and outer nuclear membranes. The microvilli were often irregular and distorted. Abnormal infected enterocytes were found scattered among enterocytes that were morphologically normal.
The histological abnormality had reverted to normal and the virus particles had disappeared in three children Bishop et al. studied after recovery some 4-8 weeks later.
The virus appears characteristically to invade the proximal small intestinal mucosa but autopsy studies have shown that abnormalities can spread along the entire length of the small intestine and even into the colon using an immunofluorescent technique (Hamilton et al., 1976).
Pathophysiology
Mavromichalis et al. (1977) found rotavirus in intestinal aspirate of 6 out of 8 children with gastroenteritis who had rotavirus in their stools. All six had abnormal xylose absorption, whereas the two without rotavirus in the lumen had normal xylose tests suggesting that small intestinal dysfunction, presumably due to mucosal damage, occurred when the virus was present in the lumen.
To date, little is known in man of the mechanism of diarrhoea in rotavirus gastroenteritis but Hamilton et al. (1976) have studied an animal model using a virus in piglets. This is a corona virus, known as the transmissible gastroenteritis virus (TGE). It produces massive diarrhoea some 16–40 hours after infection, with high faecal electrolyte levels. At 40 hours they found decreased sodium and water flux, decreased mucosal activities of disaccharidases and sodium, potassium-ATPase, but normal adenyl cyclase activity. Unlike enterotoxigenic diarrhoeas such a cholera, under these experimental conditions, sodium flux failed to respond to glucose. Ferguson and Snodgrass (1978) have gone on to study infection of 1-day-old germ-free lambs with rotavirus. They found a state of accelerated epithelial cell turnover with functionally immature enterocytes clothing the villi. If a similar situation exists in rotavirus gastroenteritis in man, it is clear that the pathophysiology may be very different from the toxigenic bacterial diarrhoeas.
Age range
Rotavirus has been found principally in the stools of children with gastroenteritis from the neonatal period up to 5 years but most are under 2 years of age. Most studies have reported a peak between 6 months and a year (Kapikian et al., 1976; Rodriquez et al., 1977), but Shepherd et al. (1975) found 43.3% of their children under 6 months.
Although the disease is commonest in young children it may also occur in adults. Rotavirus has, indeed, been found in the stools of adults with acute gastroenteritis (Von Bonsdorff et al., 1976). The virus may also occur in adults who are symptom free (Tallet et al., 1977). It is often found in the families of infected children, i.e. it is a contagious virus.
Sex ratio
As in earlier reports of non-bacterial gastroenteritis in which viral studies were not done (Gribbin, Walker-Smith and Wood, 1976; Tripp, Wilmers and Wharton, 1977), most studies of rotavirus gastroenteritis report that more boys are affected than girls particularly in younger children (Table 6.7). The reason for this male predominance is not yet clear.
Incubation period
The incubation period appears to be about 48–72 hours (Shepherd et al., 1975).
Clinical features
Most studies have described a sudden onset with vomiting as the first symptom, often accompanied by diarrhoea but sometimes preceding it by several hours (Shepherd et al., 1975). Diarrhoea is typically acute in onset and often watery in character with usually less than ten stools each day. However on occasion, there may even be no diarrhoea, with vomiting as the only symptom.
Initial clinical reports of rotavirus gastroenteritis described a relatively mild-self-limiting illness with dehydration, usually less than 5% present in about half or less of those children admitted to hospital.
Pyrexia is a common but not a constant feature. Although Champsaur et al. (1984) describe a significant association between fever, diarrhoea, vomiting and the presence of rotavirus in the stools. A concurrent upper respiratory tract infection has been documented in 42% in one report (Carr et al., 1976) and 29% in another (Rodriquez et al., 1977). Most clinical reports from developed countries have described a mild self-limiting illness with dehydration, usually less than 5%, present in about half or less of those admitted to hospital. Rodriquez et al. (1977) described dehydration in 83% of children with rotavirus gastroenteritis compared with 40% in children who did not have rotavirus in their stools. But dehydration was mostly mild to moderate. It has, however, become clear that rotavirus gastroenteritis may on occasion be associated with a much more severe illness, and death has been reported from Canada (Carlson et al., 1978) and Australia (Bishop et al., 1973). Fatal rotavirus gastroenteritis does not appear to have been documented yet in Britain although a child brought dead to Queen Elizabeth Hospital following a diarrhoeal illness had rotavirus found in his gut lumen at post-mortem. Nevertheless, it now is clear that rotavirus may be a major cause of diarrhoea-related infantile death (Carlson et al., 1978). Vesikari et al. (1983) have indeed estimated that one million rotavirus associated deaths may occur annually.
An initial study of 30 children with rotavirus gastroenteritis from the Queen Elizabeth Hospital for Children, London during the winter of 1974–1975, found a relatively mild illness (Shepherd et al., 1975). Ryder et al. in Bangladesh (1976) have indeed found rotavirus in the stools of 12 out of 22 children, with severe dehydration due to diarrhoea, the degree of dehydration being comparable with that found in cholera, in their experience.
In southern India, Maiya et al. (1976) found no difference in the clinical presentation of children with gastroenteritis in whom rotavirus was detected as compared with those in whom a bacterial pathogen was identified or no agent recognized. In rural Bangladesh when rotavirus infections were compared with most gastrointestinal infections due to other aetiological agents, rotavirus was more often found to be associated with dehydration and hospital admission. Only cholera led more often to dehydration (Black et al., 1980).
So although rotavirus gastroenteritis seems most often to cause a relatively mild illness in developed communities, it may on occasion cause more severe disease and even death. Rotavirus is a significant cause of death in the developing world.
Accompanying pathogens
Any account of the severity of the clinical disease must try to take note of the possible role of accompanying pathogens, bacterial or viral. Several reports have described a more serious illness, when bacterial pathogens, especially enteropathogenic E. coli, are found in the stools at the same time (Shepherd et al., 1975; Carr et al., 1976). Madeley et al. (1975) have found rotavirus and other bacterial and viral pathogens occurring in different stool samples from the same infants and have found it difficult to determine whether one or all were significant pathogens.
Duration of illness
The duration of illness ranges between 5 days to 3 weeks (Shepherd et al., 1975) but usually lasts only 8 days (Tallett et al., 1977). Most children present within 5 days of onset if they come to hospital.
Biochemistry and haematology
An elevated blood urea on hospital admission has been described (Rodriquez et al., 1977). Hypernatraemia may occur but is not characteristic. When there is dehydration it is likely to be isotonic. Lymphocytosis may be found and also neutropenia (Phillips, A.D., unpublished observations).
Stool microscopy
Characteristically, leucocytes are not present in the stools although they may be seen in some patients, 18% in one series (Rodriquez et al., 1977).
Infectivity
It appears to be a highly contagious disease, apparently spread by the faecal-oral route. Massive quantities of virus may be passed in the stools some 24–72 hours after infection. Outbreaks may occur in families and in institutions. In one study, 35% of parents of children with rotavirus gastroenteritis had serological or stool evidence of rotavirus infection (Rodriquez et al., 1977). It is also clear that adult staff members may play a role in transmitting rotavirus infections in children's wards.
Rotavirus in neonates
A high incidence of rotavirus in the stools of asymptomatic neonates has been reported from Sydney (Murphy, Alberry and Crewe, 1977) and St. Thomas's Hospital, London (Chrystie et al., 1975). In one study, Murphy et al. found no less than 50% of neonates by the age of 3–4 days were excreting virus. Only 24% of these had diarrhoea.
Despite the finding of rotavirus in asymptomatic neonates, Murphy et al. found that significantly more neonates with diarrhoea excreted the virus than in the symptom-free group. It does, in fact, seem clear that rotavirus can be a cause of gastroenteritis in the neonate, as suggested by Bishop et al. (1976). Quite severe symptoms have been observed in premature babies in some special care baby units (Bryden et al., 1982). The role of rotavirus antibodies in protecting the infants against rotavirus infection is uncertain. A clue to why some with stool rotavirus do not get clinical disease is provided by Murphy et al., who found that children who had in their stools viruses coated with an ‘antibody-like’ material did not get diarrhoea. It is known from the work of Matthews et al. (1976) that breast milk and cows' milk contain non-antibody virus inhibitor and this may play a role too. So neonates appear to be relatively immune to serious rotavirus infection. Rotavirus has now been described to occur endemically among neonates in hospital nurseries in a number of countries as well as England and Australia, namely the United States and Sweden (Grillner et al., 1985). In these studies rotavirus infection occurred throughout the year without any obvious seasonal variation. Another feature is the difficulty in eradicating the virus from nurseries. This may relate to the role of the staff.
Hjelt et al. (1985) have looked at rotavirus infection in the nursing staff in paediatric wards; 36% of staff in the infant/toddler wards had rotavirus. All members of the staff had rotavirus IgA or IgG antibody. As it is known that rotavirus is present in the hand-washings of children with rotavirus (Samadi et al., 1983), this study shows that nursing staff may contribute to the spread of rotavirus gastroenteritis in paediatric wards via contaminated hands.
Spread of rotavirus
A community-based study of the spread of rotavirus in 47 families in New Zealand (Grimwood et al., 1983) showed that the primary mechanism in the spread of rotavirus infection is through intrafamilial contacts. Thirty-six out of 78 adults and children exposed to children suffering from rotavirus infection developed the disease in the following 4 weeks. In contrast, none of the 53 adults and children exposed to children who had non-rotavirus gastroenteritis developed diarrhoea.
The spread of rotavirus infection has been further investigated in a prospective study using the ELISA technique in children admitted to hospital in Holland (Walther et al., 1983). The children were examined weekly for rotavirus. There was a high incidence of hospital acquired infection and even asymptomatic rotavirus excretors were identified. In another study in Paris (Champsaur et al., 1984) using stool electron microscopy and ELISA, asymptomatic excretion of rotavirus was often found to occur in children of 1–6 months of age (50%) and in those of 7–24 months (26%). The authors found only a statistical association with the diarrhoea-fever-vomiting syndrome. They subdivided rotavirus excretors into:
-
•
Rotavirus gastroenteritis.
-
•
Asymptomatic infection, i.e. rotavirus with serological response but no symptoms.
-
•
Rotavirus carriers.
All these studies pose problems for the relevance of rotavirus found in the stool to the aetiology of diarrhoea in an individual child. This probably relates to the stability of the rotavirus in the environment. It is probably spread as an aerosol to the environment during the change of nappies. Thus the main reservoir of infection is the babies themselves. In the study of Grillner et al. (1985) one single electropherotype belonging to subgroup 1 of the human rotavirus was found throughout. Hjelt et al. (1985) have studied 25 healthy Danish mothers and their breast-fed infants. All mothers had detectable concentrations of rotavirus IgA and IgG in their serum at time of lactation. These levels did not change during the period of breast feeding.
All infants had rotavirus IgG in serum by 3–4 days but none had serum rotavirus IgA or ScIg. Rotavirus IgG in the serum of infants correlated with that of their mothers. Few samples of duodenal juice from the infants contained rotavirus ScIg and IgA. On the other hand, about 80% of the infants stools samples contained rotavirus ScIg and IgA. These disappeared from the infants stool after cessation of breast feeding. So it may be inferred that infants receive rotavirus IgG through the placenta and receive rotavirus ScIg and IgA in constant amounts via breast milk during lactation. Thus any protectional effect of breast feeding requires frequent breast feeding and is limited to the period of lactation.
Diagnostic techniques
A number of techniques have been used. First and foremost has been electron microscopy of stools using negative staining, which has been used in many centres. This is a relatively simple technique provided an electron microscope is available. Second is the technique of counterimmunoelectrophoresis. This is a technique to detect viral antigen in the stool (Spence, Farrel and Bourchard, 1975; Middleton, Petric and Hewitt, 1976). It appears to be about as sensitive as the electron microscope technique. Third is serum serology using a complement fixation technique. This was developed by Kapikian, Cline and Mebus (1975). With rotavirus stool antigen, these workers showed a rise in serum antibody titre with a complement fixation test using acute and convalescent sera. A related antigen, Nebraska calf rotavirus, is more readily available. Fourth is an indirect immunofluorescent antibody technique (Davidson et al., 1975b). This is a difficult technique but one that seems to be slightly more sensitive than the complement fixation test. Fifth is the ELISA technique.
Several studies have recently looked at the comparative efficiency of various techniques for the detection of rotavirus antigens in the stools. Miotti, Eiden and Yolken (1985) compared two enzyme immunoassays (Bio Enza Bead and Rotazyme) with a latex agglutination assay (Rotalex) using solid-phase enzyme immunoassay as the reference. No false positive assay was found. All three commercial assays accurately detected rotavirus in the stools from children with rotavirus gastroenteritis but there was a decreased level of efficiency later in the course of the illness.
Another study (Julkunen et al., 1985) found latex agglutination test to be the recommended test for stool screening for rotavirus infection. Chernesky et al. (1985) have modified Rotazyme to produce Rotazyme 11. This technique achieved 99.4% sensitivity compared with electron microscopy. This was regarded as a significant advance.
Another study (Knisley et al., 1986) compared Rotazyme 11 and other polyclonal antibody-based assay systems with a monoclonal assay system which they found to be more sensitive than other assay systems. Yet another paper (Pai, Shahrabadi and Ince, 1985) studied Rotalex and compared it with direct electron microscopy and Rotazyme 1. They found it to be much faster and cheaper than electron microscopy or Rotazyme. It was claimed to be available even in the doctor's office for rapid diagnosis. It is not possible at present to adjudicate on all these techniques for field studies but what is clear is that for hospital studies the electron microscope is the technique of choice as it not only accurately shows the presence of rotavirus but accurately diagnoses other virus particles too.
Delayed recovery
In the first detailed report of the clinical features of rotavirus gastroenteritis, Shepherd et al. (1975) described temporary sugar intolerance in 2 of 30 children. In a prospective study of sugar intolerance at Queen Elizabeth Hospital of 200 children with acute gastroenteritis (Trounce and Walker-Smith, 1985) 45 children had rotavirus gastroenteritis. 15 (45%) developed sugar intolerance, 9 (20%) were monosaccharide intolerant and 6 (15%) were disaccharide intolerant. In this relatively small study there was only a statistical association between rotavirus infection and sugar intolerance. This was not found with any other virus or bacterial pathogen although the numbers were small. The glucose electrolyte solution used at the time of the study was much higher than that presently used so its importance may have been higher at that time than today. An association between sugar malabsorption and rotavirus would in fact be expected in view of the damage to microvilli in infected enterocytes (Bishop et al., 1973). In addition, it would appear theoretically possible that severe and extensive infection could temporarily damage a high proportion of lactase activity of the enterocytes and, perhaps, also cause a transient surface block to sugar absorption by temporarily damaging the brush border membrane which would cause a block in absorption to all sugars, as proposed by Walker-Smith, Kamath and Stobo (1972). The clinical severity would depend on the number of enterocytes damaged. Finally, Holmes et al. (1976) have suggested that lactase is the receptor and uncoating enzyme for rotavirus. This would mean that infants with their high lactase levels may be more vulnerable, helping to explain the age of most affected individuals.
Immunity
Little is known yet about immunity to rotavirus infection. Repeat infection with rotavirus in childhood has rarely been reported (Fonteyne, Zusis and Lambert, 1978; Simhon et al., 1981). In the former report a different type was responsible for the second infection but not in the latter. Reinfection was also reported in the Santa Maria Caque study. Antibody levels tend to rise with age, reaching adult levels at two years (Kapikian et al., 1976). Children with pre-existing antibody have become infected, however. Infection with one subgroup may not protect against another. Infants aged 6-24 months appear more susceptible to infection regardless of the presence of antibody in their sera. Lower prevalence rates of rotavirus infection under 6 months may be attributable to immunity acquired from the mother. Bortulussi, Szymanski and Hamilton (1974) found normal amounts of IgA in intestinal secretions during acute illness and in convalescence.
Protection against rotavirus
It is clear that there is an urgent need to provide protection against rotavirus. Vesikari et al. (1983) have tested an oral live rotavirus vaccine RIT 4237 of bovine origin for immunogenicity and safety in adults and subsequently in children. They also showed in a randomized double-blind placebo controlled trial, the ability of this vaccine to protect against natural rotavirus infection in children. A single dose of RIT 4237 was given to 178 infants aged 8–11 months (Vesikari et al., 1984). During 5 months observation after vaccination two of the 86 vaccine recipients and 18 of the 92 placebo recipients had rotavirus diarrhoea lasting more than 24 hours which was clearly a significant result. No side-effects attributable to the vaccine were observed. The two children who were not protected were primary vaccine failures as they had no detectable serum antibody responses after vaccination. It remains to be seen whether this vaccine will provide protection against all subgroups of human rotavirus. The epidemic in Finland, at the time when protection was found, was caused by subgroup 2 rotaviruses.
An effective vaccine for rotavirus should have an important effect upon the very high mortality rates for children from gastroenteritis in the developed world (De Zoysa and Feachem, 1985). As already pointed out, bovine (RIT 4237) vaccine has been shown to be safe and effective against rotavirus gastroenteritis in Finland (Vesikari et al., 1985).
However, it has had a disappointing lack of effectiveness in developing communities (De Mai et al., 1986), e.g. in Rwanda. This failure could be due to over-attenuation of the virus and interference by other enteric viruses affecting children in developing communities where multiple viral infection is common. Another rotavirus vaccine derived from rhesus monkey rotavirus RMV (MMU-18006) has been studied in Venezuela as it is more immunogenic (Flores et al., 1987). The protective efficiency of RMV vaccine against rotavirus diarrhoea was 68% of 247 infants aged 1–10 months studied for up to 1 year. More interestingly, for the entire study group, vaccine efficiency was 100% against the most severe rotavirus diarrhoeal episodes. Most rotavirus strains detected in the study belonged to rotavirus subgroup 2 which includes serotypes 1, 3 and 4. Protection afforded was limited to subgroup 2 viruses.
This study represents a most encouraging result and clearly further extensive field studies are indicated.
Rotavirus-like agents
A number of rotavirus strains which are antigenically distinct from rotavirus causing human gastroenteritis have been recognized. These are called rotavirus-like agents (RVLA). They have been isolated from a wide range of animals and also man. These non-group A rotaviruses have been tested for in infants and adults with gastroenteritis by oral inoculation of infant rats with stool specimens from 16 patients. Six faecal specimens, but no other disease controls, gave rise to infection in the rats (Eiden, Vonderfecht and Yolken, 1986). All these rats had antigenic evidence of infection as determined by immunofluorescent staining of their gastrointestinal epithelium.
Vonderfecht et al. (1985) studied an enzyme immunoassay inhibition assay for the detection of rat rotavirus-like agent in intestinal and faecal specimens from both rats and humans with diarrhoea. Four of six human faecal specimens that induced diarrhoea in suckling rats were positive for this assay system.
This rotavirus-like agent has a similar RNA migration pattern to a rotavirus-like agent described in adults in China. This virus, now known as ADRV, has been further studied by counterimmunoelectrophoresis (CIE) as a method for detecting antibodies to this virus from healthy adult subjects in China, Hong Kong and Australia. No outbreaks of diarrhoea due to this virus have been yet reported from Hong Kong or Australia yet 18% and 15% respectively were found in two outbreaks in Junzhou, China where this virus was first identified (Tao et al., 1985). This antibody was also found in 47% house rats, 36% pigs and 17% wistar rats. These observations thus raise the possibility of animal origin for this virus infection in man. Flewett (1986) has pointed out that a good study to determine on the one hand whether animal rotaviruses do infect young children or on the other whether human rotaviruses infect young animals is badly needed.
Norwalk-like agents
The Norwalk agent was the first stool virus to be identified as a cause of gastroenteritis. It is a 27 nm small structured virus. There are in fact now several other Norwalk-like agents which are morphologically similar but may be antigenically distinct. These include the Hawaii agent, the Montgomery country agent and the Snow Mountain agent. However, the relationship between these agents is not yet clear. The story began in October 1968 with an outbreak of acute non-bacterial gastroenteritis in a school in Norwalk, Ohio. The symptoms consisted primarily of nausea and vomiting but diarrhoea and a low-grade pyrexia were also noted. The disease lasted 24 hours and remitted spontaneously (Adler and Zickl, 1969). A bacteria-free filtrate was prepared from the rectal swab of an adult secondarily infected during the outbreak. Administered orally, this produced acute gastroenteritis in adult volunteers (Dolin et al., 1971). A 27 nm virus-like particle was subsequently found in a stool filtrate prepared from an adult volunteer, which had been aggregated by convalescent sera using the technique of immune electron microscopy (Kapikian et al., 1972). Using this technique of immune electron microscopy of the stools, virus particles of similar morphology but probably antigenically distinct have been identified in other outbreaks of gastroenteritis. The Hawaii agent was detected in a family outbreak in Hawaii in 1977 (Thornhill et al.). Then in 1979 a 27-32 nm virus particle was identified in a large outbreak of gastroenteritis in Granby, Colorado (Morens et al., 1979) called the Snow Mountain Agent.
Despite the antigenic variation of these particles, their morphological similarity has led them to be classified together as the Norwalk-like group of viruses. An interim classification scheme for small round human faecal viruses based primarily on size and morphology was described by Caul and Appleton in 1982. It is now possible for these Norwalk-like particles to be identified in the stools by electron microscopy and on the basis of structure alone to be distinguished from similar small structured viruses, i.e. astro virus and calicivirus, and from featureless small round viruses by an expert electron microscopist (Figure. 6.18 ). This technique of immune electron microscopy by means of which these virus particles were first recognized, also permitted antibody titres to be demonstrated and thus provided evidence of an association between these agents and clinical gastroenteritis. However, immune electron microscopy is a cumbersome, labour intensive technique and subsequently a sensitive and efficient radioimmunoassay for Norwalk agent (Greenberg et al., 1981) was developed. A similar assay has been developed for Snow Mountain agent (Dolin et al., 1986).
Figure 6.18.
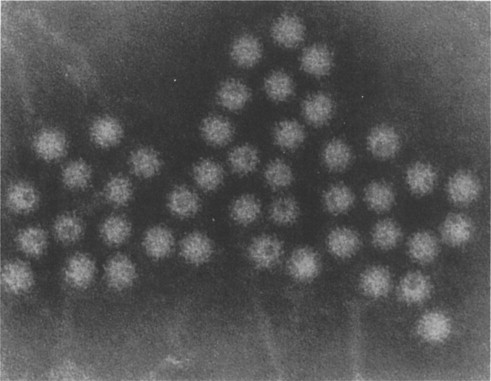
Norwalk-like particles in stool demonstrated by electron microscopy.
(Reproduced by kind permission of Phillips.)
A great deal of information about these agents has come from adult volunteer studies as no animal model exists and as yet there is no in vitro culture system (Dolin, Treanor and Madore, 1987). So at present the evidence that Norwalk-like viral particles have an aetiological association with gastroenteritis may be summarized as follows:
-
•
Oral administration of material containing the particles regularly induces illness in normal volunteers.
-
•
The viral particle is present in the stools at the time of illness and not before.
-
•
Rises in titre of serum antibody to the particle can be demonstrated in acute and convalescent serum samples from volunteers given the agent, and also individuals with naturally occurring disease.
-
•
Rises in titre of serum antibody to the particle in general do not occur when adults given the particles do not become ill.
More recently, Treanor et al. (1986) have developed a monoclonal antibody to the Snow Mountain agent, the first monoclonal antibody for this group of agents. If this can be developed also for the Norwalk-agent this will facilitate the development of practical detection assays.
In Western societies its clinical importance in children has been uncertain, mainly related to past difficulties in accurately diagnosing this particle on stool electron microscopy. These have now been overcome and since 1982 it has been possible to recognize Norwalk-like agents by their morphology. From the literature the Norwalk agent has in fact been associated chiefly with outbreaks of gastroenteritis. Older children and adults have most often been affected. One very large community outbreak was reported when approximately 3000 people developed an illness compatible with Norwalk gastroenteritis after consuming bakery items with frosting (Kuritsky et al., 1984).
Outbreaks of Norwalk gastroenteritis have been associated with drinking contaminated water supplies and recreational lake water, and eating contaminated food such as oysters and salad.
Indeed eating raw shellfish has long been associated with individual and sporadic cases of gastroenteritis. In the past it has often been impossible to identify a cause. However, during 1982 outbreaks of gastroenteritis associated with eating clams and oysters reached epidemic proportions in New York State. Norwalk agent was implicated as the principal aetiological agent in no less than five of seven outbreaks on the basis of seroconversion and the development of IgM antibody to Norwalk virus. In addition, Norwalk virus was identified by radioimmunoassay in clam and oyster specimens from two of the outbreaks although not in the few stools examined (Morse et al., 1986). The relative importance of the Norwalk agent in food-borne associated outbreaks awaits evaluation; clearly it can on at least some occasions be a most important cause.
Clinical features
Sporadic cases of Norwalk
Both gastroenteritis and hospital acquired infection can occur. A prospective study was undertaken at Queen Elizabeth Hospital for Children of all children admitted to the gastroenteritis unit between March 1982 and September 1983, who had Norwalk-like agent in their stools as well as all children who developed acute gastroenteritis while on other wards in the hospital who had this agent in their stools. Small round structured viruses with the features of Norwalk agent were found in 42 specimens from 28 patients from a total of 1360 stool specimens. The children who had Norwalk-like agent in their stool were divided into one third who were admitted with acute gastroenteritis and two thirds who developed acute diarrhoea after admission for another reason (Storr et al., 1986). Those who were admitted with gastroenteritis and who had Norwalk-like agent in their initial stool specimen had mild symptoms only.
Those who developed diarrhoea after admission, i.e. had nosocomial or hospital acquired infection, also had a mild illness. 17 children had Norwalk-like agent alone in the stools and 11 had other viruses as well.
Vomiting was present in only 8 of the 17 children in whom Norwalk-like agent was identified on its own. The vomiting was mild, lasting 1–3 days, and was more common in those whose admission was for gastroenteritis. This mildness of vomiting as a symptom is in contrast to the earlier reports of Norwalk agent causing outbreaks of winter vomiting disease.
The diarrhoea was likewise mild, and settled rapidly with a glucose electrolyte solution which was prescribed in most cases, but in seven children the symptoms were so mild that no treatment at all was given.
Symptoms in those children with multiple viral infections were also not severe, except for the three with combined rotavirus, Norwalk-like agent and astro virus infections. Two of these developed delayed recovery failing their initial regrade back onto cows' milk feeds but later tolerated cows' milk feeds. However, a third child developed cows' milk sensitive enteropathy and was discharged on a casein hydrolysate formula. Interestingly significant sugar intolerance was not seen in any children in this study.
Age ranged from 9 weeks to 2 years with an average of 8 months which is younger than earlier reports.
The seasonal incidence was different from rota virus. During the study period there was a spring peak, but this has not been consistent since that time. Certainly there is not a winter peak.
Adenoviruses
Adenoviruses are 75 nm in size and icosahedral in shape (Figure. 6.18) with a characteristic morphology.
Adenoviruses may be found in the stools of children with acute diarrhoea and may be present in large numbers even though the adenovirus is not often cultured from tissue culture of these stools. The evidence that these adenoviruses are in fact pathogenic in these circumstances is again based on ‘guilt by association’ (Flewett, 1976a), but as earlier workers in this field established, adenoviruses may be found in the stools of apparently well controls. These enteric or fastidious adenoviruses identified by electron microscopy in the stools of children with diarrhoea which are difficult to cultivate have unique antigenic properties when tested with ELISA. Two distinct serotypes Ad 40 and Ad 41 have been identified. Johansson et al. (1985) have developed an ELISA for direct detection of enteric adenovirus 40 and 41 in the stools. This assay proved to be specific. It is a sensitive and rapid technique. Kidd et al. (1987) report another technique, namely a dot-blot hybridization test allowing direct detection of fastidious enteric adenovirus DNA in stool specimens.
Clinical features
Non-cultivatable or enteric adenoviruses have been associated with outbreaks of diarrhoea and vomiting in children (Richmond et al., 1979) more so than adenoviruses which can be grown in culture. There has been a long-standing uncertainty about an aetiological role for adenoviruses in gastroenteritis. There are reports, however, of death related to adenovirus where it was found in the mucosa of a child who died from gastroenteritis (Whitelaw et al., 1977). In addition adenovirus has been detected by electron microscopy in the mucosa of a child who suffered a sudden unexpected death (Phillips, 1982).
Further evidence that adenovirus may cause infantile gastroenteritis (Figure. 6.19 ) comes from a report that an outbreak of gastroenteritis was due to a newly identified subgroup of adenovirus designated as F and closely related to type 40 adenovirus (Chiba et al., 1983).
Figure 6.19.
Adenovirus particles in stool demonstrated by electron microscopy.
(Reproduced by kind permission of Phillips.)
Coronavirus
Coronavirus are large (80–400 nm) pleomorphic enveloped RNA viruses (Figure. 6.20 ). They are known to cause acute gastroenteritis in piglets and calves and were first reported in human faeces in Vellore, India (Mathan et al., 1975) and then from Bristol (Caul, Paver and Clarke, 1975) in young adults as well as from children with acute diarrhoea.
Figure 6.20.
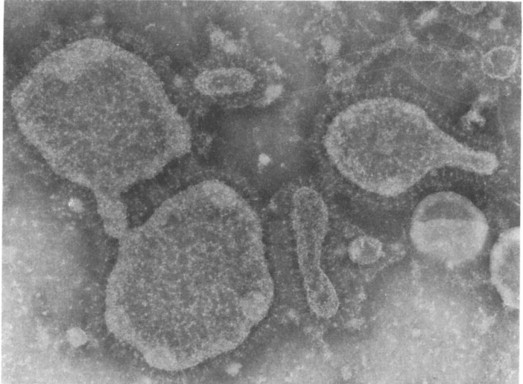
Coronavirus particles in stool demonstrated by electron microscopy.
(Reproduced by kind permission of Phillips.)
Human coronavirus is now accepted as a definite morphological entity, even though doubt has in the past been cast on the ability to recognize this virus in the stools. Its electron-microscopic features have now been well defined.
The role of coronavirus in the causation of a mild coryzal illness and acute respiratory infections has been clearly established, and it has been implicated in the aetiology of some cases of pneumonia, although the evidence for this is less strong. Its bovine counterpart has been well defined as one of the causal agents of calf enteric disease. Gastroenteritis has also been experimentally induced in piglets by intragastric inoculation of this virus. Its role as a cause of human gastroenteritis is not yet clear. In a study of the stools of asymptomatic subjects it was not found in any of a small number (30) of a control paediatric population attending the outpatients at Queen Elizabeth Hospital (Weindling et al., 1980). It was found, however, in 5.6% of asymptomatic adults (Clarke et al., 1979) in South West England and over 90% in southern India of stools of children and adults (Mathan et al., 1975).
Clinical features
Coronavirus was sought in the stools of all children admitted to Queen Elizabeth Hospital with acute gastroenteritis between 1st January 1980 to 1st August 1983; 18 . of 2387 stool specimens (0.6%) had the coronavirus in 15 children by electron microscopy. Coronavirus was detected in stools more frequently in the spring and summer months, 12 out of 15 cases occurring between April and August. In six patients, other enteric pathogens were also present: two had Shigella, one entero-pathogenic E. coli, one rotavirus, and two Giardia lamblia.
It was found predominantly in older children. Only two out of 15 were less than 1 year old. Eleven were older than 2 years. This is a striking contrast to rotavirus gastroenteritis. There was a high incidence of children of immigrants (12 out of 15 (80%) compared with the usual rate of 52% for children with gastroenteritis admitted to Queen Elizabeth Hospital). In eight out of fifteen cases (53%) there had been recent travel abroad, either to the Indian subcontinent, or to Africa, compared with 5.3% in the gastroenteritis unit as a whole, i.e. the most important feature of these patients was the high incidence of those who had recently returned from tropical or subtropical areas. The most likely explanation for this finding is that it reflects the high incidence of coronavirus excretion in the areas from which they have returned; in other words, a transplanted third world population is being examined. Although it remains unexplained why these children are asymptomatic whereas coronavirus is found so often in asymptomatic children in India.
Only six patients had no other medical conditions or associated intestinal pathogens. These children had an episode of acute self-limiting diarrhoea of mild severity only. Two patients were pyrexial, although the temperature never rose above 37.9°C. No reducing substances were found in the stools of any child with coronavirus. Further work is required to determine the importance of this virus.
Astrovirus
Another small virus particle found on electron microscopy of stools of children with gastroenteritis has been designated the astrovirus because of its star-like appearance (Madeley and Cosgrove, 1975); it is 28–30 nm in diameter (Figure. 6.20). Astro viruses too have been detected in the stools of animals including sheep, cattle and mice. A stool filtrate from a child with astrovirus gastroenteritis has been administered to eight normal volunteers (Kurtz et al., 1979). Although these volunteers shed large amounts of virus only one of them developed vomiting and diarrhoea. Thus astrovirus appears to be less pathogenic in these volunteer studies than does Norwalk agent when administered in a similar way.
Astroviruses have been difficult to culture in vivo (Lee and Kurz, 1981). They have only been detected immunologically by immune electron microscopy or by immunofluorescence of infected tissue cultures. There appear to be at least five serotypes (Kurtz and Lee, 1984). Rise in antibody titre has been found in natural disease using immune electron microscopy.
Astrovirus has been proved to be an enteropathogen in the lamb by Snodgrass et al. (1977) who fed astrovirus particles to gnotobiotic lambs and produced mild diarrhoea at a time when astroviruses were in the stools or intestinal contents. Astroviruses were also demonstrated in infected enterocytes and there was an enteropathy with depression of lactase activity in the mid-small intestine. Phillips et al (1982). found astrovirus in mucosa of a child at Queen Elizabeth Hospital who also had astrovirus in the stools at the same time (Figure. 6.21 ).
Figure 6.21.
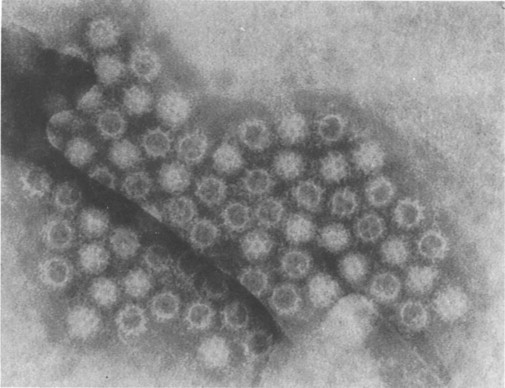
Astrovirus particles in stool demonstrated by electron microscopy.
(Reproduced by kind permission of Phillips.)
Clinical features
From among 1248 stool specimens examined during the 2-year period, February 1979 to February 1981 at Queen Elizabeth Hospital astrovirus was detected by electron microscopy in 42 stool specimens from 28 children (Nazer, Rice and Walker-Smith, 1983). Diarrhoea was an invariable clinical feature. Vomiting occurred in 18 children, abdominal pain in 7, and mild dehydration in 5, i.e. less than 5% dehydration. Fourteen of the children with acute diarrhoea were admitted directly to the gastroenteritis unit. The other fourteen children developed their acute diarrhoea some time after hospital admission. Although the excretion of astrovirus was associated with mild gastroenteritis, the presence of other enteric pathogens in 16 of the 28 children limited the degree to which the clinical symptoms could be attributed to astrovirus alone.
Excretion of astrovirus alone was found to be less common in summer than in winter. Excretion of astrovirus with rotavirus showed a winter peak.
Examination of serial stool specimens from some children showed that astrovirus excretion occurred for at least 7 days (Kurtz et al., 1977). In relation to the date of onset of diarrhoea, astrovirus was detected for at least 8 days in three children while in another the stool became negative for astrovirus 4 days after onset of diarrhoea. Transient monosaccharide intolerance lasting 1–2 days occurred in 18 children, and cows' milk protein intolerance requiring milk elimination for several months was a sequel in three children.
This experience then confirms that astrovirus occurs in hospital acquired infections and sporadic gastroenteritis. It has been detected in school outbreaks too and in nursing home outbreaks. On balance it seems probable that astrovirus is a human enteric pathogen causing mild diarrhoea and vomiting in children.
Calicivirus
Another small virus particle has been identified with a characteristic morphology as calicivirus (Figure. 6.22 ). It is difficult to grow but Cubitt and Barrett (1985) have succeeded in growing calicivirus in human embryonic kidney cells. Using immune electron microscopy there appear to be four or five serological types of human calicivirus. A rise in the titre of antibody to Norwalk agent has been found to be caused by these agents (Dolin et al., 1987). The relationship of these two viruses awaits clarification.
Figure 6.22.
Calicivirus particles in stool demonstrated by electron microscopy.
(Reproduced by kind permission of Phillips.)
Caliciviruses have a characteristic appearance under the electron microscope consisting of a scalloped border with cup-shaped indentations on the surface. They were first described by Madeley and Cosgrave in 1976 in the stools of children with gastroenteritis. They were also found in an outbreak of winter vomiting disease in a school in London (Cubitt et al., 1979) as well as from the small bowel of a child who died from gastroenteritis (Flewett and Davies, 1976).
Calicivirus is associated with a mild diarrhoeal illness, often hospital acquired. Calicivirus infection has since been chiefly associated with community outbreaks and nosocomial infection but sporadic cases have also been described.
The relative frequency for the identification of the individual viral particle at Queen Elizabeth Hospital is indicated in Table 6.2.
Breda-like virus
A pleomorphic virus-like particle approximately 100 nm in diameter with a fringe of closely applied peplomers (7-9 nm in length) was observed by electron microscopy in the stools from 20 children and adults with gastroenteritis (Beads et al., 1984). Under electron microscopy these virus-like particles resembled the Breda virus isolated from calves with diarrhoea. Whether these particles will prove to be human pathogens awaits elucidation. Interestingly, two of the patients had the haemolytic uraemic syndrome.
Small round viruses
There remains a final group of viruses for which little information is available. These small round structureless viruses when examined with the electron microscope range in size from 20–30 nm in diameter. They have been found in a variety of sporadic cases and also in outbreaks (Kogasaka et al., 1980).
Bacterial gastroenteritis syndromes
Shigellosis
Chantemesse and Widal in France, in 1888, were the first to associate the shigellae with dysentery but a more complete description was given in Japan by Shiga in 1898. Shigellosis is an acute enterocolitis due to infection with an organism of the shigella group. The term shigellosis is not synonymous with bacillary dysentery, which is infection with Shigella shiga. Other Shigellae are 5. sonnei, S. flexneri and S. boydii. Shigellosis is a highly contagious infection, requiring a very low infecting dose. Transmission is by person-to-person contact and food/water contamination by human faecal matter, man being the only natural host although flies may serve as vectors.
Clinical features
Approximately 25% of patients will have mild watery diarrhoea, an equal number will have high fever with relatively minor enteric complaints, and the remainder will progress through a period of diarrhoea accompanied or followed by fever and, in a day or two, cramping abdominal pain, tenderness and severe diarrhoea containing blood and/or mucus. Straining to defaecate may be so severe as to cause rectal prolapse but vomiting is infrequent.
Children under the age of 10 years are particularly severely affected, perhaps due to their poorer sanitation practices or diminished coproantibodies. Furthermore, in younger children, the abrupt rise in body temperature may be accompanied by prostration, meningismus or febrile seizures. Acute symptoms may persist for a week or more, with convalescence prolonged over several additional weeks. Although dehydration and hyponatraemia are common in severe forms, septicaemia and complications are otherwise rare.
Diagnosis
The peripheral white cell count is often elevated but regardless of the acute number of cells, there is a predominant number of polymorphonuclear cells and their precursors. Examination of the rectum may reveal an oedematous and friable mucosa with ulcers present. Rectal prolapse may occur. Stools usually demonstrate many red and white cells on smear. Laboratory stool culture can be difficult and the sample should be plated out immediately, as a delay of 2 hours decreases recovery by 50%.
Species identification in the United Kingdom will usually demonstrate S. sonnei or S. flexneri. S. shiga (S. dysenteriae, the original Shiga bacillus) and 5. boydii are far less common. Serotype identification is used in epidemiological control.
Treatment
The most important consideration is replacement of fluid and electrolyte losses. Antibiotics should be restricted to fulminant infections in debilitated infants. Shigella is sensitive to many antimicrobials, including trimethoprim-sulphamethoxazole (treatment of choice), but the organism is now often resistant to ampicillin. The importance of hygiene should be emphasized. In countries where good sanitary facilities exist and where water is available for hand washing, morbidity from Shigella infection is low. Even in developed nations in situations where personal hygiene is diminished, as in day-care centres and custodial institutions, outbreaks of shigellosis have occurred, often involving numerous secondary cases.
Salmonellosis
There are more than 1000 types of this Gram-negative bacillus that make up the genus salmonella. Infection with these organisms may cause a variety of illness in animals and in man.
Infection may result in several distinct clinical entities including acute gastroenteritis, septicaemia, focal infection, and the enteric fevers. The enteric fevers, caused by S. typhi and S. paratyphi are exclusive to man and present primarily as septicaemic disorders. In contrast, the other types of salmonella which are zoonoses most commonly result in disease localized to the gastrointestinal tract, i.e. acute gastroenteritis, although septicaemia with metastatic focal infection including meningitis, osteomyelitis, pneumonia, endocarditis, endopthalmitis and cholecystitis may occur, particularly in infancy.
Many infections with salmonellae in children arise from animal sources via contamination of food but food may also be contaminated by the infected stools or urine of a person who is excreting salmonellae. The risk of hospital cross-infection with salmonella infections is considerable, because of this infectivity of stools and urine. Small numbers of organisms can cause illness in a compromised host but very large numbers may have no effect in a healthy naturally immune host (Curry, 1976).
Salmonella infections are common in farm animals, particularly poultry. The increasing use of frozen foods, especially poultry, in developed countries may be an important factor in the observed trend of an increase of salmonellosis in such countries (Fenner, 1971). While infection with salmonella has been considered of greatest importance to children in developing countries salmonella food poisoning is a disease of modern western society.
Interestingly, there was a higher percentage of children with gastroenteritis who were found to have salmonellae isolated from their stools in Sydney (see Table 6.1), where the trend to increased poultry consumption is more advanced, than in the East End of London where children had salmonellae isolated. However, in fact there is now an overall trend in England and Wales of increasing reports of salmonella infections. This relates to an increase in food poisoning incidents due to serotypes other than S. typhimurium and from 1980 to 1984 an increase in incidents due to S. typhimurium. There was one notable outbreak in 1985 when S. ealing was found in dried milk powder from one manufacturer which probably originated from one cow in a herd supplying milk to the factory; 62% of reported cases were in infants less than 1 year of age and one died.
Incorrect cooking methods, for example inadequate cooking of incompletely thawed frozen poultry, are responsible for such infections. Salmonella is endemic in Britain and many foods both home-produced and imported are contaminated. Table 6.20 indicates some recent outbreaks. It clearly is a serious matter with death resulting in some cases. The incubation period is 12-36 hours.
Some types of salmonella infection occur both in animals and in man whereas others such as typhoid fever due to Salmonella typhi occur only in man. The small intestine is significantly involved in typhoid fever as, characteristically, the lymph follicles of Peyer's patches are inflamed and as a result ulceration may occur. Uncommonly in children, but more commonly in adults, perforation and haemorrhage may result. However, both typhoid fever and paratyphoid fever are primarily septicaemic diseases that manifest primarily as systemic disease, but on occasion a child admitted to a gastroenteritis unit may have this organism cultured from his stool.
The other species of salmonella cause predominantly a gastrointestinal disorder, but occasionally blood stream infection may occur and in neonates this is more common than in older infants. Neonates may even develop meningitis due to a salmonella organism. Other situations where blood stream infection may occur include sickle cell disease and chronic granulomatous disease. In both conditions osteomyelitis may develop and indeed may be the clinical mode of presentation of the salmonella infection.
This vulnerability of the neonate to blood stream infection with salmonella may be related to low levels of IgM in the neonatal period. This is particularly likely as it is known that antibodies against the O antigen of Gram-negative organisms are chiefly found in IgM. This may also account for the susceptibility of young infants to infection with enteropathogenic strains of E. coll. Penetration of small intestinal, particularly ileal, or colonic mucosa by this ‘invasive’ organism results in fluid secretion and diarrhoea which may be mediated by prostaglandins released secondary to inflammation (Bachino, Suchy and Snyder, 1984).
Most salmonellae have two main antigens, the H or flagellar antigen and the O or somatic antigen. Some have a third antigen known as the Vi antigen, e.g. Salmonella typhi. These various antigenic components may be used to identify the particular species of salmonella. Their number, as mentioned earlier, is large but Salmonella typhimurium is the commonest encountered in most series. Table 6.21 indicates the types of salmonellae encountered in children with gastroenteritis at the Royal Alexandra Hospital in 1970.
Table 6.21.
Gastroenteritis survey Royal Alexandra Hospital for Children 5 Jan. 1970-3 Jan. 1971, 838 admissions salmonellosis (53 cases)
| Salmonella typhimurium | 34 |
| Salmonella derby | 3 |
| Salmonella adelaide | 3 |
| Salmonella chester | 2 |
| Salmonella bovis morbificans | 2 |
| Salmonella anatum | 1 |
| Salmonella singapore | 1 |
| Salmonella kottbus | 1 |
| Salmonella newport | 1 |
| Salmonella muenchen | 1 |
| Salmonella bredeney | 1 |
| Salmonella senftenberg | 1 |
| Not typed | 2 |
Over 500 children presented to the Queen Elizabeth Hospital for Children in London with salmonella infections of any kind over a 12-year period from 1973 to 1984 (Figure. 6.23 ). A substantial increase in the total number of infections from 1978 can be seen but infection due to 5. typhi and S. paratyphi has not increased over this time. During this period no deaths were attributable to salmonella infection.
Figure 6.23.
Annual numbers of children with Salmonella at Queen Elizabeth Hospital, 1973–1984.
There has been a more than threefold increase in salmonella as a cause of inpatient gastroenteritis over the twelve years, from 1.9% in 1973 to 2.2% in 1977 and 7.4% in 1984. A similar trend was seen in the number of salmonella isolates in all stools examined at this hospital including inpatients and outpatients in 1973; 10 out of 4912 (0.2%) stools examined contained salmonella species. This increased to 26 out of 4900 (0.5%) in 1977 and 47 out of 7390 (0.63%) in 1984. Thus in addition to an absolute increase in the number of salmonella infections of any kind, this organism is now responsible for a larger proportion of both inpatient gastroenteritis and positive bacterial isolates in the stools.
Clinical features
In the 12 months between October 1983 and 1984, 56 children presented with salmonella infections to the Queen Elizabeth Hospital and fifty were studied in detail prospectively (Elliott et al., 1987). Thirty were male and 20 female and at presentation the age was 5 weeks to 13 years. In this skewed distribution the median age was 9 months and the mean 21.7 months; 66% of the patients were less than 12 months of age and 10% less than or equal to 3 months of age. The occurrence of enteric fever was unrelated to age, although numbers are small (Figure. 6.24 ). Asian children were over-represented in these children with salmonella infections. All enteric fevers occurred in children of Asian immigrants. Paratyphoid fever was acquired in Pakistan by one child, and in India by the other. Typhoid fever occurred in two children who had not left the country but had contacts who had recently acquired typhoid in Pakistan and Bangladesh. Two children acquired non-typhoid salmonella infections during visits to their countries of origin, Italy and Mauritius.
Figure 6.24.
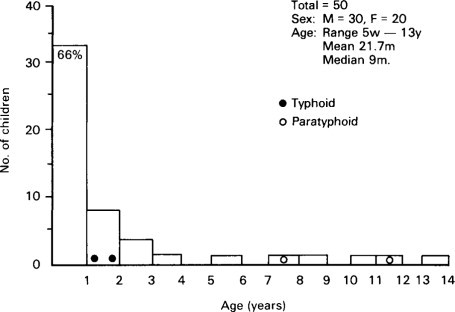
Age of 50 children with Salmonella infections at Queen Elizabeth Hospital.
The presenting features in patients with non-typhoid salmonella infections and in those with enteric fever is shown in Table 6.20. Diarrhoea, vomiting and fever were the most frequent in both groups. In contrast, enteric fever in adults is more often associated with constipation than diarrhoea. A number of children with non-typhoid salmonella infections presented with a ‘septicaemic’ picture, a high swinging fever, cough or abdominal pain. This resulted in several misdiagnoses at presentation, including appendicitis, pneumonia and intussusception. Both children with S. typhi, but neither with S. paratyphi had a swinging fever. Only two children were more than 5% dehydrated and one with typhoid had rose spots.
Of the type of salmonella isolated in the 50 children S. typhimurium was the most common. Two children had S. typhi (phage types 5 and 28) and two had S. paratyphi. A salmonella was grown from the stools in all children although three—with S. Newport, S. enteritids and S. typhimurium—had no gastrointestinal symptoms, possibly because the acute illness was days to weeks before presentation. Two children had an additional bacterial pathogen (Campylobacter and the Aeromonas species) in the stool and 4 had rotavirus. S. typhi and S. infantis were grown from the urine in two (4%) children and S. infantis in one (2%) from the CSF. Blood culture was performed in 10 children who were ill with a persistently high or swinging fever. Five (10%) children had positive blood cultures. The majority of positive cultures occurred in infants. Of the two children with 5. typhi, one had a positive blood culture, but neither of those with S. paratyphi had a positive culture. This may reflect the time at which blood was taken in relation to the course of the illness.
The enteric fevers have almost disappeared in developed communities where safe water supplies exist and the majority of cases diagnosed in the United Kingdom are acquired overseas, particularly in the Indian subcontinent. Reports show that this amounts to over 80% of typhoid and paratyphoid A and over 55% of paratyphoid B seen in the UK since 1975 (Galbraith, 1985). Infection acquired in this country may also result from contacts who have travelled abroad. The occurrence of enteric fever exclusively in Asian children in this study reflects this ethnic group's practice of returning to their homeland for visits (Hutchins et al., 1982) but highlights the fact that the enteric fevers may occur in persons who have never left the United Kingdom.
The particular susceptibility of the infant to salmonella infection was confirmed in this study in which 66% were less than 12 months and 10% less than or equal to 3 months of age. This may be due to immature host defence mechanisms including poorly developed mucosal defence which is improved by breast feeding but impaired in the malnourished or premature infant. Intrafamilial transmission of salmonella is an important mode of acquisition in infancy and the incidence of complications in this age group is high.
The study indicates that salmonella is a common and continuing cause of infective diarrhoea in children in East London. Further, enteric fever may occur. Consideration of this diagnosis must be given in any child with fever of unknown origin, accompanied by cough or diarrhoea.
Treatment
Antibiotic treatment of uncomplicated salmonella gastroenteritis may prolong the carrier state and increase the incidence of relapse and is not recommended unless there is accompanying septicaemia or metastatic infection when sensitivities will determine the antibiotic used. Chloramphenicol for 10-14 days remains the drug of choice for the enteric fevers when the organism is sensitive (McKendrick, Geddes and Farrell, 1982). Ampicillin, co-trimoxazole and trimethoprim are also effective. Carriers should be treated with ampicillin and probenecid for 6 weeks initially; however, cholecystectomy is occasionally required if this is unsuccessful. Salmonella meningitis requires a minimum of 3 weeks appropriate antibiotic therapy following negative CSF culture.
Some authors suggest antibiotic treatment for all infants less than 3 months of age who have salmonella isolated from the stool (Raucher et al., 1983). Although the complication rate of salmonellosis is high, and a septic screen is performed when indicated, antibiotic therapy should be reserved for infants with clinical or bacteriological evidence of septicaemia or focal infection outside the gastrointestinal tract.
There are a number of ways to limit and prevent salmonella infection. These include good animal husbandry including prevention of entry of infected animals into flocks. The final line of defence, however, is good kitchen hygiene. Undercooking, inadequate cooling, the use of contaminated processed foods and takeaway meals are all important.
Escherichia coli enteritis
This subject has been well reviewed by Boedeker and Sherman (1986). Adam, in Germany, in 1923, identified special types of lactose splitting strains of E. coli as a cause of severe infantile gastroenteritis (Braun, 1974) but this observation was not widely accepted, and up until 1945 shigellae and salmonellae were the only bacterial pathogens to have been established as causes of infantile gastroenteritis. In that year, Bray at the Hillingdon Hospital in London published observations he had made concerning the isolation of enteropathogenic strains of E. coli from infants with summer diarrhoea. It has subsequently been widely confirmed that certain strains of E. coli are enteropathogenic. The criteria for accepting such enteropathogenicity usually includes the consistent isolation of a particular strain in an epidemic as the predominant coliform in the stools of affected infants and its relative rarity in the general community. The antigenic structure of each strain is recorded and reference criteria are developed for future recognition.
Several types of antigen are assessed in identifying the strains of E. coli. These include the O, K and H antigens. It is the O antigen that is particularly important in identifying enteropathogenic strains of E. coli. This antigen is a heat-stable lipopolysaccharide associated with the bacterial cell wall. A number of B subtypes are recognized. The enteropathogenic strains known at present are as follows:
| 026 | 044 | 055 | 086 | 0111 |
| 0112 | 0114 | 0119 | 0125 | 0126 |
| 0127 | 0128 | 0142 |
These strains of E. coli may colonize the small intestine, and their capacity to do so is an important factor in determining their enteropathogenicity. This appears, in turn, to be related to the ability of these organisms to adhere to the surface mucosa (McNeish et al., 1975).
Another critical factor in determining their enteropathogenicity appears to be the elaboration by some strains of E. coli of a diarrhoea-producing enterotoxin (Orskov et al., 1976). Enterotoxigenic E. coli have been shown to produce two types of toxin. First, there is the heat-labile (LT) enterotoxin that is antigenically and functionally similar to cholera toxin. It stimulates adenyl cyclase, as does cholera enterotoxin, and also produces an excess loss of water into the intestinal lumen, i.e. a secretory state. The second toxin is the non-antigenic heat-stable (ST) toxin whose mode of action is via stimulation of another intracellular mediator (cyclic GMP) (Field, 1978). Our understanding of these mechanisms of enteropathogenicity have been considerably expanded by animal work, notably that of Williams Smith (1976). It has now been shown from such studies that the ability of E. coli to colonize the gut, adhere to mucosal surface, and to produce enterotoxin can be transmitted from one organism to another by transmissible plasmids. These are extra-chromosomal genetic elements. Thus plasmids could transfer the potential to produce one of these capacities, e.g. enterotoxin production, to organisms not ordinarily regarded as pathogens. At present, enterotoxin can be demonstrated only by its effect on animal models or on cultured cells. The development of a simple laboratory procedure that would allow rapid recognition of such an enterotoxin would be a great advantage and might demonstrate the Enteropathogenicity of hitherto unsuspected organisms.
Another critical factor that may determine the enteropathogenicity of E. coli strains is the ability of the organisms to invade the epithelial cells of the intestine. Du Pont and his colleagues (1971) suggested that two different types of clinical illness could occur, enterotoxin-producing strain or an invasive strain. In the former, severe watery diarrhoea similar to that seen in cases of cholera occurred, whereas in the latter an illness similar to shigellosis occurred.
The relative importance of enteropathogenic, enterotoxin-producing and invasive strains of E. coli in Britain remains uncertain. Although enterotoxin producing strains of E. coli that cause a severe disease with profound fluid loss, sometimes referred to as cholera infantum, may occur such disease is far more common in the developing world than in developed communities. The best evidence that these mechanisms of toxin production and invasion are causes of human disease comes from studies of E. coli strains that cause traveller's diarrhoea in adults. In fact, most studies of classical enteropathogenic E. coli serotypes have failed to show that those strains, associated with sporadic or epidemic cases of infantile diarrhoea, possess either invasive or toxin-producing properties. Levine et al. (1978) have shown that three enteropathogenic strains (0127, 0128, 0142) given to adult volunteers all cause diarrhoea, but despite this there is no evidence of toxin production or invasiveness of these strains in extensive laboratory studies. There is evidence in fact that the classic E. coli enteropathogenic organisms produce their effects by a completely different mechanism described as entero-adherent E. coli of the cytotoxic variety (Clausen et al., 1982) (Figure. 6.25 ).
Figure 6.25.
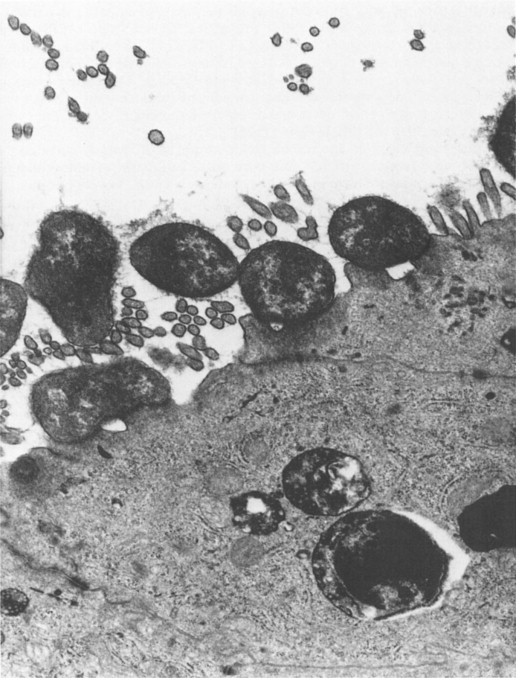
Entero-adherent E. coli as visualized with electron microscope, adherent to enterocytes × 21 200.
(Reproduced by kind permission of Phillips.)
Neonatal infection with an enteropathogenic strain of E. coli is particularly dangerous and can spread very rapidly through a nursery of newborn infants and be associated with a very severe form of diarrhoea and even death. The significance of the isolation of such a strain during an epidemic of infantile gastroenteritis is quite clear but its isolation from the stool of the occasional symptom-free infant is uncertain. Nevertheless, the isolation of such a strain from the stools of a child with diarrhoea under the age of 2 years is usually regarded as a significant finding.
Thus, to say the least, from the viewpoint of the practising clinician the present position concerning diagnosis and recognition of infection due to strains of enteropathogenic E. coli is most unsatisfactory. It is clear that invasive and toxin-producing strains of E. coli do exist and are not detected by conventional serotyping. Conventional serotyping continues to be valuable (Boedeker and Sherman, 1986). The author recommends the continuation of the present routine serotyping of E. coli.
Ironside (1973) has speculated that the disease may be due to the temporary clinical upset that occurs when new strains of E. coli reach the gut of the infant. A similar change is considered to be one of the causes of ‘travellers’ diarrhoea’. He suggests that a ‘baby's trip to the local nursery is the bacteriological equivalent of the adult's trip to Egypt’. The incubation period for infections with these strains is believed to be 24 to 72 hours.
Table 6.1 indicates the isolation rate for these organisms in four hospitals in different countries. Within Britain itself there has been a change since 1953 when the MRC trial of prophylactic antibiotic found that 37% of children with gastroenteritis were infected with one of the only two serotypes then known to be enteropathogenic. This figure is higher than the present figure of 9.5% at the Queen Elizabeth Hospital and the figure reported by Ironside, Tuxford and Heyworth in Manchester in 1970, namely 4%. These findings suggest a continuing change in the aetiology of gastroenteritis. Infection with enteropathogenic E. coli is associated with a high incidence of the post-enteritis syndrome.
Yersiniosis
The genus Yersinia contains three Gram-negative cocobacillary organisms pathogenic to man, Y. pestis, the plague bacillus, Y. enterocolitica and Y. pesudotuberculosis. The term yersiniosis is usually reserved for disease caused by the last two.
Epidemiology
The incidence of Y. enterocolitica infection is more common especially in Scandinavia and Belgium too than in Britain. Serotypes 03 and 09 are prevalent in this area. The organism has been isolated from wild and domestic animals, water supplies and a number of foods. Two large outbreaks due to contaminated milk have been documented in the USA.
Y. pseudotuberculosis is wordwide in distribution and has been recovered from wild and domestic animals. Man probably acquires the infection by contact with animals. Most cases are sporadic and occur in the winter months.
Pathogenesis
Y. enterocolitica causes mucosal ulceration in the terminal ileum and less commonly colon, necrotic lesions in Peyer's patches and enlargement of mesenteric lymph nodes. The appendix is usually normal. Y. pseudotuberculosis causes purulent mesenteric adenitis with microabscess formation, granulomata and occasional giant cells.
Clinical features
In children it presents usually as acute gastroenteritis in younger children and as acute terminal ileitis or mesenteric adenitis in adolescents (Vantrappen et al., 1977).
The most commonly recognized form of yersiniosis is a relatively mild self-limiting enteritis. This is more common in children less than 5 years of age and is usually caused by Y. enterocolitica. Fever, diarrhoea, abdominal pain and less commonly vomiting last from 1 to 3 weeks. The stools may contain leucocytes and sometimes blood and mucus. Chronic diarrhoea occasionally simulates chronic inflammatory bowel disease (see Chapter. 9). In children over 5 years old abdominal pain is a common complaint and often mimics acute appendicitis. Fever and leucocytosis are common while diarrhoea may or may not be present. Terminal ileitis, commoner with Y. enterocolitica, and mesenteric adenitis, commoner with Y. pseudotuberculosis, are found at laparotomy. Those who escape surgery frequently develop a right iliac fossa mass which usually subsides with adequate treatment. Pharyngitis may accompany the gastrointestinal presentation. Serious disease, especially septicaemia, is uncommon in healthy children and is usually associated with immuno-compromised hosts, cirrhosis, diabetes and haematological disease. Thalassaemics are particularly susceptible as they are iron overloaded, and being treated with desferrioxamine therapy, factors which favour yersinia. In common with other living organisms, nearly all bacteria require iron for growth and usually acquire it by producing sideophores which are low molecular weight compounds with a high affinity for ferric iron (Neilands 1981). In mammals, the bacteria must compete for available iron with lactoferrin or transferrin. The bound iron is then internalized by specific membrane receptors allowing the bacteria to multiply in tissues (Finkelstein, Sciortina and Mcintosh, 1983).
Yersinia is unable to produce its own siderophores but can utilize iron in siderophores from alternative sources including other bacteria e.g. enterobacteria in the gut (Perry et al., 1979). Desferrioxamine is a potent siderophore and hence its clinical application in patients with iron overload. It is likely that yersinia has receptors for desferrioxamine and can utilize the bound iron directly, thus increasing its virulence. In mice with yersinia infections, prior administration of desferrioxamine reduced the median lethal dose 100 000 fold compared with mice pre-treated with iron dextran (Robins-Browne et al., 1985). In addition, continued administration of desferrioxamine led to yersinia septicaemia in adults with iron-overload and in normal children with iron poisoning (Melby et al., 1982).
In a case at Queen Elizabeth Hospital described by Kelly et al. (1987) continued administration of desferrioxamine exacerbated yersiniosis in a thalassaemic child and contributed to her septicaemia by encouraging the systemic spread of yersinia which is normally confined to the bowel.
The mortality rate of yersinia septicaemia is very high (around 50%). Although rare it is more common in immuno-compromised patients or those with iron overload or cirrhosis.
A number of immunological complications occur (Kohl, 1979; Foberg et al., 1986). Reactive arthritis is the most common in adults (up to 30%). This complication may persist for months and is associated with HLA B27. It is less common in children.
Erythema nodosum in children with yersiniosis is usually due to Y. pseudotuberculosis and is more common in boys. Rheumatic fever and glomerulonephritis may also be associated with yersiniosis.
Diagnosis
Clinical suspicion and close co-operation between clinician and laboratory are important factors in diagnosis.
Yersinia pseudotuberculosis is very difficult to culture from stools. It can be cultured from blood or mesenteric lymph nodes, especially those in the ileocaecal angle (Mair and Fox, 1986). Yersinia enterocolitica is easier to culture from stools. Again blood or lymph nodes may yield the organism (Kohl, 1979).
Serology is not very useful in the acute stage in Yersinia enterocolitica but may be in Yersinia pseudotuberculosis (Mair and Fox, 1981). Positive titres usually disappear in a few months but may persist for years especially in patients with arthritis.
Despite the similarity of this illness to Crohn's disease, there appears to be no evidence that yersinia infections ever result in Crohn's disease. The ulceration of the ileum and colon heal within a few weeks.
Barium studies may show abnormalities of the terminal ileum which could be confused with Crohn's disease, but Vantrappen et al. (1977) state that the radiological differential diagnosis is not difficult. Radiological abnormalities are mainly changes in mucosal pattern. The superficial ulceration and skip lesions of Crohn's disease are never seen.
Autopsy studies show that the entire alimentary tract may be involved by an ulcerative process, primarily at the site of lymphoid tissue. Apthoid ulcers may be seen in colon similar to Crohn's disease but no sarcoid granulomata or giant cells are ever found.
Treatment
In otherwise healthy children self-limiting enteritis should be treated symptomatically as for other causes of gastroenteritis (Kohl, 1979; Mair and Fox, 1981).
Terminal ileitis and mesenteric adenitis should be treated with appropriate antimicrobials. Septicaemia requires intensive antimicrobial and supportive therapy (Shapiro, 1981; Kattamis et al., 1984). Y. enterocolitica produces B lactamase and penicillins are usually inactive. Chloramphenicol, augmentin, gentamycin, co-trimoxazole and third-generation cephalosporins have been used successfully although monotherapy with any of the last three is occasionally associated with relapse. Augmentin with the addition of chloramphenicol in seriously ill patients appears to be the best choice. Yersinia pseudotuberculosis is usually sensitive to ampicillin, chloramphenicol, cefotaxime, co-trimoxazole and others.
A case has been made for prophylaxis or rather early empirical treatment of thalassaemic children presenting with fever and enteritis or pharyngitis. Kruger et al. (1985) reported good results using co-trimoxazole. In thallasaemics desferrioxamine should be stopped in the acute phase of the disease (Kelly et al., 1987).
Campylobacter enteritis
Campylobacter is a Gram-negative motile bacterium which was first described in 1913 (MacFadyean and Stockman) and was placed in the genus vibrio. In 1963 the alternative name Campylobacter was used as this term means curved rod in Greek, which describes the shape of these bacteria. The most common species are Campylobacter jejuni and Campylobacter coli which cause disease in man. More rarely Campylobacter fetus and Campylobacter laridis may cause infection in immunocompromised patients. It has been claimed that Campylobacter pyloris may cause gastritis. It was only in 1972 that Dekeyser et al. first associated Campylobacter infection with human disease. This was largely related to the fact that previous culture techniques were unsatisfactory.
It is now known to be a common cause of bacterial diarrhoea in the United Kingdom and is a common stool pathogen in acute gastroenteritis at Queen Elizabeth Hospital for Children at present (Table 6.20). Worldwide it is very common (Billingham, 1981). Transmission of infection from human to human is unusual and most often transmission is from animals to man. Human infection is a zoonosis. Sources of infection include frozen poultry, wild birds, milk and domestic pets (Robinson, 1981).
Clinical features
A ‘flu like prodrome may occur occasionally with no gastrointestinal symptoms followed by the onset of diarrhoea and abdominal pain often colicky in nature. This abdominal pain may vary from mild to very severe even suggesting an acute abdomen. There is often blood in the stools which may be confused with intussusception. Much less often Campylobacter infection may be associated with haemolytic-uraemic syndrome, toxic megacolon, bacteraemia and even Guillian-Barre syndrome. There are also asymptomatic carriers of Campylobacter (Skirrow, 1977).
Pathology
Both. jejunum and ileum may be inflamed also with acute inflammation of the mesenteric lymph nodes. The colon may also be involved. The principal mechanism of disease is invasion based on clinical and experimental evidence and also secretion. Enterotoxin-mediated diarrhoea has been reported in children (Blaser et al., 1979) but toxin production is not necessarily related to clinical expression of infection.
Treatment
As with all gastroenteritis the mainstay of treatment is fluid replacement. Antibiotic therapy, usually erythromycin, has also been used in severe cases. If given in the first four days such treatment does cut duration of symptoms and faecal excretion of organisms. It was reported to be life-saving in cases of bacteraemia and toxic megacolon (Kalkay et al., 1983). Antibiotics curtail the carrier state rather than prolonging it. Sometimes outbreaks of gastroenteritis in day-care centres are due to infection with Campylobacter. The problem of reinfection by the same agent, sometimes called the ping-pong mechanism, may be a problem in this situation. This is caused by the prolonged excretion of Campylobacter which can last up to 7 weeks (Karmali and Fleming, 1979). The benefits of treatment with erythromycin estolate 50 mg kg−1 daily in children with positive cultures leading to successful eradication of the infection has been described by Ashkenazi et al. (1987).
Aeromonas infection
Aeromonas spp. are found in soil and natural water sources. They have been recognized for many years as a cause of disease in non-mammalian species including red leg disease of frogs and black rot in hen's eggs, but they have also been found in mammals viz, fish and pigs. Gracey et al. 1982 reported aeromonas-associated gastroenteritis in children. Further reports of aeromonas as a potential cause of acute diarrhoea in children have since been published (Kipperman et al., 1984; Ljungh et al., 1985; Nazer et al., 1986). Aeromonas has also been described as a cause of traveller's diarrhoea (Gracey et al., 1984; Taylor et al., 1985).
Clinical features
A clinical study of children with Aeromonas spp. admitted to Queen Elizabeth Hospital April to September 1984 was undertaken (Nazer et al., 1986). The children had an acute diarrhoeal illness of short duration, sometimes with blood in the stools. Aeromonas spp. were isolated from 4.1% of children who had stools examined during the study period. Their age ranged from 2 months to 7.5 years, 33 children had Aeromonas spp. identified, 16 had enterotoxin producing Aeromonas hydrophilia, nine enterotoxin producing Aeromonas sobria and eight non-entero-toxin producing Aeromonas caviae. Gracey et al. (1982) suggest that non-entero-toxin producing organisms are not a significant cause of gastroenteritis. Six children had associated pathogens, bacteria or viruses. Five patients had recently returned from abroad, chiefly from developing countries. The clinical associations of the 25 children who had no other pathogen is shown in Table 6.22 . Four children had bloody diarrhoea. Only six patients were admitted to hospital. Thus aeromonas infection is rarely associated with serious sequelae, other than in the immunosuppressed patient. There are reports, however, of clinical features similar to ulcerative colitis (Gracey et al., 1982).
Table 6.22.
Clinical associations of aeromonas spp. in children's stools
| Clinical features | No. of children (%) (25) |
|---|---|
| Diarrhoea | 21 (84%) |
| Vomiting | 10 (40%) |
| Abdominal pain | 9 (36%) |
| Fever >38°C | 4 (16%) |
| Dehydration (mild) | 2 (8%) |
Aeromonas spp. were isolated from four children who did not have diarrhoea. There have been reports in the past of Aeromonas in asymptomatic children (Von Graevenitz et al., 1986). As no control stools were examined during the study period the true role of Aeromonas is still not yet clear. Several workers regard it as a definite cause of diarrhoea but others are less certain. Prospective studies are still required.
Treatment is usually fluid therapy but occasional cases with prolonged diarrhoea require antibiotic therapy and co-trimoxazole may be effective for some patients.
Food-poisoning organisms
When acute diarrhoea and vomiting rapidly follow the ingestion of contaminated food, the diagnosis of food poisoning is made. Most often it is due to bacterial contamination although viruses such as Norwalk agent as pointed out earlier may also cause it.
Food poisoning may result from ingestion of bacteria per se or preformed toxin, e.g. staphylococcal enterotoxin, as well as the toxin of Clostridium botulinum (classic botulism), fortunately a rare occurrence today. Clostridium perfringens continues to be an important cause of food-borne diarrhoea and can cause a fatal enteritis known as ‘pig-bel’ in the highlands of New Guinea. Classically, infection with salmonella may produce this syndrome but other organisms have now been associated with food poisoning. These include Vibrio parahaemolyticus and Bacillus cereus.
Vibrio parahaemolyticus is a Gram-negative rod associated with the consumption of uncooked sea foods. Its incubation period is 12-48 hours. Acute gastrointestinal symptoms last a few days but rarely are serious.
Bacillus cereus is a Gram-positive rod that can produce a toxin. Infection is most often acquired by eating food that has been stored before eating, e.g. Chinese fried rice.
Complications of gastroenteritis
Apart from the acute disturbances of water, electrolyte and acid-base balance that may complicate gastroenteritis, there are a number of problems that may follow acute gastroenteritis. These include problems at the time of regrading and also long-standing problems associated with chronic diarrhoea and failure to thrive. These complications are listed in Table 6.23 .
Table 6.23.
Complications of acute gastroenteritis
| Immediate |
| Dehydration |
| Electrolyte imbalance: |
| Hypernatraemia |
| Hyponatremia |
| Hypokalaemia |
| Acid–base disturbance: acidosis |
| Protein losing enteropathy |
| On initial regrading: |
| Monosaccharide intolerance |
| Disaccharide intolerance |
| Chronic diarrhoea (post-enteritis syndrome) |
| Food intolerance syndromes: |
| monosaccharide intolerance |
| disaccharide intolerance |
| cows' milk protein intolerance |
| Chronic diarrhoeal syndromes of unknown aetiology: |
| may be associated with persistent small intestinal |
| mucosal damage and alteration of bacterial flora |
| (quantitative and qualitative) |
Protein-losing enteropathy is not a common complication, but when it occurs it is usually brief and self-limiting. It may temporarily cause diagnostic confusion with other causes of protein-losing enteropathy such as small intestinal lymphangiectasia (see page 77).
The syndromes of sugar intolerance may manifest as a brief self-limiting problem at the time of initial regrading, but sometimes may persist. At other times the syndrome may present later, sometimes as part of a more severe syndrome with chronic diarrhoea and failure to thrive.
Food intolerance has been known for some time to be a sequel to gastroenteritis. Sunshine and Kretchmer (1964) described fat and sugar malabsorption as sequelae of gastroenteritis. Burke, Kerry and Anderson, in 1965, emphasized the importance of lactose intolerance as a cause of refractory watery diarrhoea in infancy. They described a group of infants who had transient lactose malabsorption following clinical gastroenteritis in association with an abnormal small intestinal mucosa and low lactase levels. In relation to protein intolerance, transient gluten intolerance and temporary cows' milk protein intolerance have been described following gastroenteritis. These two syndromes are discussed in Chapter. 5.
Sugar and/or fat malabsorption may manifest immediately after gastroenteritis. As a result, the term post-gastroenteritis malabsorption has been used to describe this condition, yet clinically detectable malabsorption may not always be present despite the presence of chronic diarrhoea and failure to thrive following an attack of acute gastroenteritis. It is therefore suggested that the term post-enteritis syndrome is a more useful term when defined as follows.
The post-enteritis syndrome is the clinical syndrome when a child who has had an attack of acute gastroenteritis subsequently has intermittent or chronic diarrhoea for more than 2 weeks with or without failure to gain weight following the return to a normal diet. In clinical practice two main groups of problems cause delayed recovery after acute gastroenteritis in infancy. First, there is an acute intolerance to the increasing concentration of milk; and secondly, there is a more chronic problem with persistent diarrhoea and failure to thrive.
Delayed recovery after gastroenteritis
Terminology
Most often acute gastroenteritis is an acute self-limiting illness and after 24 hours of a glucose electrolyte solution followed by a regrade to the infant's usual cows' milk based feeding formula there is full recovery. Some looseness of stools may persist for a few days. However, there is no worsening of diarrhoea, return of vomiting or further risk of dehydration and weight gain is satisfactory, once there is adequate calorie intake. Thus there is uneventful recovery. In some infants recovery is delayed. Most often this may present as an intolerance to milk as this is reintroduced into the child's diet i.e. there is a failed re-regrade or a clinical relapse requiring the child to return to a glucose electrolyte solution or even require an intravenous infusion. When such delayed recovery occurs up to 2 weeks after the commencement of oral rehydration therapy it is likely to be part of the natural history of the gastrointestinal infection as most cases of gastroenteritis especially those of viral origin are associated with pathogen excretion in the stools for no more than 10 days to a fortnight. Hence during this period any clinical problem with relapse of symptoms may be embraced by the umbrella term delayed recovery. Although this term does extend beyond this period, once 2 weeks have elapsed then persistence of symptoms must be regarded as being part of the post-enteritis syndrome. This then must be regarded as a problem extending beyond the expected period of spontaneous recovery. It must be remembered, however, that in the cases of some bacterial syndromes of gastroenteritis, for example shigellosis, the natural history of the disease may often extend for several weeks.
Clinical spectrum
Delayed recovery then is seen in the clinical context of initially a child failing a regrade during the period of the acute infection itself and in the later situation of a child having persistent symptoms following this acute episode for more than 2 weeks. The criteria for failing a regrade may be continuing severe diarrhoea due to the presence of secretory diarrhoea or more often the return of diarrhoea, i.e. on return to cows' milk i.e. intolerance to milk.
With acute intolerance to milk there is a return of diarrhoea, which is often watery and copious and sometimes accompanied by vomiting. This syndrome is most often due to malabsorption of lactose and sometimes also of sucrose, but there may also be intolerance to cows' milk protein (see Chapter. 5). Sometimes, in addition, there may be monosaccharide intolerance (see Chapter. 7). It is often brief in duration but it may also persist. These acute syndromes when lasting up to 2 weeks may be regarded as part of the natural history of the acute infection.
Fat malabsorption may occur; indeed, some degree of temporary steatorrhoea is a frequent accompaniment of gastroenteritis. This can be demonstrated indirectly by estimating plasma vitamin A (a fat-soluble vitamin) at the time of acute episode of gastroenteritis and after recovery. This will show a low level, and rise to normal on recovery (Araya et al., 1975), although factors other than vitamin A malabsorption may also play a part in this change in vitamin A blood levels, such as decreased mobilization of stores. Sivakumar and Reddy (1972) have shown impaired absorption of labelled vitamin A acetate in gastroenteritis, and steatorrhoea may also be demonstrated by a conventional fat balance.
The more chronic problem of persistent diarrhoea and failure to gain weight following gastroenteritis may be more difficult to diagnose. It may be confused with coeliac disease when the child's diet includes gluten. Indeed, the differential diagnosis of any child under 2 years with chronic diarrhoea and failure to thrive is an important problem. In these circumstances the following diagnoses need to be considered, namely post-enteritis syndrome, coeliac disease, cystic fibrosis, giardiasis and cows' milk sensitive enteropathy as well as an anatomical abnormality of the small intestine. Chronic diarrhoea without failure to thrive following acute gastroenteritis needs to be distinguished from toddler's diarrhoea in which there is chronic diarrhoea but no failure to thrive (see Chapter. 12). Sometimes follow-up is the only way to establish the diagnosis definitely. Small intestinal biopsy has a key role in diagnosis. When symptoms have been present for 3 weeks or more after gastroenteritis, and there is doubt about the diagnosis, a small intestinal biopsy should be considered. This will demonstrate whether there is any structural abnormality of the small intestinal mucosa that may account for the child's continuing symptoms (Figure. 6.26 ). Such mucosal damage (small intestinal enteropathy) may be a sequel to acute gastroenteritis or relate to another disease entity such as coeliac disease.
Figure 6.26.
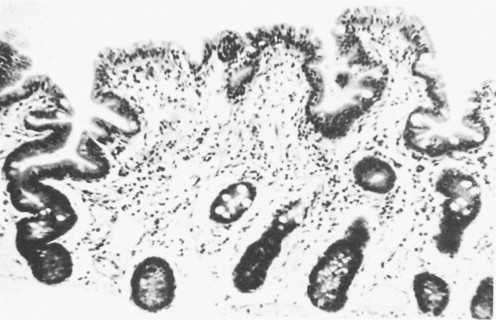
Partial villous atrophy occurring as a sequel to gastroenteritis.
Indeed if the mucosa is flat, the diagnosis of coeliac disease must be considered provided the child is eating gluten, even though it is known that gastroenteritis per se may cause a flat mucosa. An intra-epithelial lymphocyte count and gliadin antibody estimation may be useful. Only reinvestigation at a later date after a period on a gluten-free diet, as outlined in Chapter. 4, may enable the final diagnosis to be made.
Why such persistent mucosal damage occurs in some children after gastroenteritis yet not in others is at present the subject of investigation since most children who develop acute gastroenteritis have a short illness from which they recover quickly. Gribbin, Walker-Smith and Wood (1976) analysed the clinical factors that may predispose to the development of delayed recovery in a prospective study of the problem at the Queen Elizabeth Hospital in 1973. They found delayed recovery occurred in 74 of the 348 children admitted to the gastroenteritis unit who survived acute gastroenteritis (21.2%) and went on to analyse the clinical factors that appeared to predispose to delayed recovery.
The prevalence of delayed recovery in 214 children under 2 years of age with simple acute gastroenteritis (see page 205) admitted to the unit in 1973 is indicated in Figure. 6.27. This shows that delayed recovery was a problem confined to infants under 18 months of age and particularly to those under 6 months. The largest group was under the age of 3 months at the time of the acute attack. In this youngest age group there was a clear male predominance. Analysis of more recent experience between 4th October 1976 and 20th January 1978 (Manuel, 1978) has shown a comparable prevalence of delayed recovery in the age group under 6 months, namely 30% compared with 27% in 1973. There has, however, been a reduction in the total number of children admitted under 6 months, and a reduction particularly in those admitted under 3 months of age. The reason for this welcome change parallels the increase in breast feeding in the community and also the change in artificial feeding from high solute milks to low solute milks (see page 22) between 1973 and 1977 (Table 6.23). Nevertheless, delayed recovery is more likely to occur in infants who develop gastroenteritis under 6 months, and it is also more likely to be a more serious problem within that age range.
Gribbin, Walker-Smith and Wood (1973) also found that there was a higher incidence of delayed recovery in infants whose weight on admission, corrected for dehydration, was below the 3rd percentile. This was related to the ethnic origin of the children concerned (Table 6.24 ).
Table 6.24.
Incidence of delayed recovery according to weight* on admissions
| Ethnic origin |
I Below 3rd percentile |
II 3rd–50th percentile |
III Above 50th percentile |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | NR | Total | DR | NR | Total | DR | NR | Total | DR | NR | Total | |
| North European | 5 | 15 | 20 | 15 | 72 | 87 | 6 | 43 | 49 | 26 | 130 | 156 |
| Asian | 7 | 5 | 12 | 11 | 15 | 26 | 1 | 7 | 8 | 19 | 27 | 46 |
| Negro | 1 | 2 | 3 | 3 | 24 | 27 | 4 | 7 | 11 | 8 | 33 | 41 |
| Mediterranean | 1 | — | 1 | 2 | 3 | 5 | — | 3 | 3 | 3 | 6 | 9 |
| Total | 14 | 22 | 36 | 31 | 114 | 145 | 11 | 60 | 71 | 56 | 196 | 252 |
| Percent delayed recovery | 39% | 21% | 15% | 26% | ||||||||
| Chi-square | I-II:4.73 | II-III:1.05 | I-III:7.3 | I-II+III:6.75 |
| (P) | (0.1<P<0.05) | (n.s.) | (P<0.001) | (P<0.01) |
DR: delayed recovery; NR: normal recovery.
Weight on admission corrected for dehydration; children with birthweights under 2500 g or twins were excluded.
Thus the incidence of delayed recovery was more than doubled when the weight was under the 3rd percentile compared with those over the 50th percentile. Weight on admission reflects nutrition. So nutrition appeared to have an important influence. Nevertheless, there were 11 children who developed delayed recovery whose weight was above the 50th percentile on admission.
The relationship of delayed recovery to ethnic origin in their study as a whole is indicated in Table 6.25 . Apart from a numerically small mediterranean group, the Asian infants, that is infants originating from the Indian sub-continent, had the highest incidence of delayed recovery. It is likely there may be both a genetic and nutritional reason for this. Asian children have a high incidence of lactase deficiency, and undernutrition is more common in this group.
Table 6.25.
Relationship of delayed recovery to ethnic origin
| Ethnic origin | Total No. of children with acute gastroenteritis |
Delayed recovery following acute gastroenteritis |
|
|---|---|---|---|
| No. of children | % ethnic total | ||
| North European | 208 | 48 | 23.0 |
| Asian | 72 | 24 | 33.3 |
| Negro | 58 | 10 | 17.2 |
| Mediterranean | 12 | 7 | 58.3 |
| Mixed parentage | 17 | 4 | 23.5 |
| Total | 367 | 93 | 25.3 |
Table 6.26 summarizes factors that may predispose to delayed recovery. The categories of delayed recovery recognized in the study of Gribbin et al. are listed in Table 6.27 . Persistent diarrhoea was a consistent feature of all three categories but disaccharide intolerance was a simple and easily resolved problem (see Chapter. 7). The other two categories with persistent diarrhoea, some of whom also had evidence of sugar intolerance, and were a more difficult and intractable problem, were grouped together as the post-enteritis syndrome.
Table 6.26.
Possible factors influencing delayed recovery after gastroenteritis
| 1. Type of feeding—breast or bottle—influencing age of presentation and size of problem |
| 2. Age at time of acute gastroenteritis, influencing severity and size of problem; severer problems under 6 months. |
| 3. Nutrition; undernutrition may predispose. |
| 4. Ethnic group may predispose—?nutrition ?genetic. |
| 5. Sex. Male sex more vulnerable in early infancy. |
Table 6.27.
Categories of delayed recovery (Gribbin et al., 1976)
| 1. Disaccharide intolerance | 38 |
| 2. Prolonged diarrhoea | 14 |
| 3. Failure to thrive | 36 |
Sugar intolerance
In Gribbin's study of infants prospectively observed after acute gastroenteritis, 9.9% developed disaccharide intolerance. Table 6.28 indicates the relationship to ethnic origin. It is clear that Asian infants were most often affected. There was a wide variation in time interval before diarrhoea with excess reducing substances appeared, after there was a return to a milk-containing diet. Most often the interval was a week or less, but sometimes it was as long as a month.
Table 6.28.
Disaccharide intolerance related to ethnic origin
| Ethnic origin | Total No. of children |
Children showing disaccharide intolerance (children receiving special feeds in brackets) |
|
|---|---|---|---|
| No. | % of ethnic total | ||
| North European | 208 | 16(11) | 7.7 (5.3) |
| Asian | 72 | 15 (9) | 20.8 (12.5) |
| Negro | 58 | 4 (3) | 6.9 (5.2) |
| Mediterranean | 12 | 1 (1) | 8.3 (8.3) |
| Mixed parentage | 17 | 2 (2) | 11.8(11.8) |
| Total | 367 | 38 (26) | 10.3 (7.0) |
This study did not take account of monosaccharide intolerance i.e. to the oral glucose-electrolyte mixture with excess stool reducing substances. From the same unit Manuel et al. (1984) did describe the importance of transient monosaccharide intolerance as an important complication of acute gastroenteritis. Strictly speaking such monosaccharide intolerance is most often a feature of the acute illness per se and does not persist beyond 3 weeks after the onset of acute diarrhoea. On occasion, however, as Manuel et al. point out it may be more persistent and is then part of the post-enteritis syndrome and may then be associated with food sensitive enteropathy.
Thirty one infants (4%) in their study from a total of 720 had clinically significant monosaccharide intolerance complicating acute gastroenteritis. Rotavirus was detected in 64% of these infants in whom it was sought. Thus monosaccharide intolerance was chiefly associated with rotavirus gastroenteritis.
In a more comprehensive study, again from Queen Elizabeth Hospital, Trounce and Walker-Smith (1985) studied prospectively 200 consecutive children admitted with acute gastroenteritis for the prevalence of sugar intolerance. A total of 31 children (15.5%) were diagnosed as sugar intolerant by the technique described in Chapter. 7: 8% were monosaccharide intolerant, i.e. glucose intolerant; 7.5% were disaccharide intolerant, i.e. lactose intolerant. All except one were under 2 years of age. Most of the children with disaccharide intolerance were under 6 months whereas only two of the children with monosaccharide intolerance were under 6 months.
There was no clear explanation for this. There was a strong association with rotavirus; 51.7% of those with sugar intolerance had rotavirus in their stools compared with 21% of the remaining sugar tolerant children with acute gastroenteritis. The frequency and importance of monosaccharide as a clinical problem relates to the glucose content of the oral rehydration solution (Kjellman et al., 1982). In the above studies all children were managed with a glucose-electrolyte solution of high glucose content (i.e. 5 g 100 ml−1). Thus in studies where lower glucose levels are used such as the WHO solution this is likely to be a much less common problem.
Comparing the development of disaccharide intolerance i.e. lactose intolerance between Gribbin's and Manuel's study an interesting and important difference occurred in relation to the time interval between the reintroduction of milk and appearance of lactose intolerance in the two studies. It was far earlier in Manuel's study. In the former, infants were fed high-solute milks and in the latter modern adapted milks during the recovery period. There is evidence that the high solute milks are more sensitizing than are the modern adapted milks both from animal studies (Anderson et al., 1979) and from clinical studies (Manuel and Walker-Smith, 1981). Thus the disaccharide intolerance described by Gribben is likely to have been largely secondary to persistent damage due to cow's milk sensitization. This is discussed in more detail in Chapter. 5.
The disaccharide intolerance that occurs closer to the onset of acute gastroenteritis is more likely to reflect small intestinal mucosal damage due to the presence of infection per se. Like monosaccharide intolerance in these circumstances lactose intolerance may be regarded as part of the natural history of the infection itself. This argument is supported by the further fall in frequency of lactose intolerance as a complication of acute gastroenteritis at the Queen Elizabeth Hospital for children. In the most recent survey (Armistead et al., 1987) of patients seen in 1986, there was no case of clinical sugar intolerance (although the glucose concentration of the GEM was significantly lower). Admittedly, these were all mild cases of gastroenteritis. This was also found in a Finnish report of the same year (Isolauri et al., 1986). Likewise the observation from North America (Groothuis et al., 1986) that replacement of lactose with either sucrose or glucose polymer in a soy-based formula offered no advantage is consistent with this view of the diminishing importance of lactose intolerance in mild gastroenteritis in Western societies (Lancet, 1987). This is probably due to the decline in cows' milk sensitive enteropathy as an important complication of gastroenteritis due to the decreased sensitizing capacities of modern adapted milk feedings.
Role of infection
The role of infection in children with failed regrades was evaluated prospectively at Queen Elizabeth Hospital from October 1982 to July 1983 (Phillips and Walker-Smith, 1986). The study included 37 out-patients and 32 in-patients who represented all cases of delayed recovery from 334 admitted (i.e. 10%). This figure contrasted with a figure of 24% found in Gribbin's study. A surprisingly high percentage of these failed regrades were associated with the presence of infective organisms in the stools (80%). Most of these were rotaviruses and 67% of virus positive stools had rotavirus. However, 27% of children with uneventful regrades also had stool pathogens. The difference was statistically significant.
In 21 of 31 in-patients the infection appeared to have been hospital acquired; most were rotavirus. In the remainder there appeared to be persistence of infection as the cause of the problem.
Eighteen failed regrades were due to lactose intolerance, 16 were associated with stool pathogens, 12 with rotavirus. Indeed in this study lactose intolerance was most often associated with infection either persistent or hospital acquired. Like the study of Trounce and Walker-Smith (1975) most cases occurred within a few days of initiating a regrade. Thus the concept emerges of lactose intolerance within 2 weeks of acute diarrhoea being due to infection per se and lactose intolerance occurring after more than 2 weeks being related to cows' milk sensitive enteropathy. Clearly both disorders overlap. At present lactose intolerance has reduced in frequency due to decline in cows' milk sensitive enteropathy and what remains is usually relatively brief in duration and related to infection per se.
Post-enteritis syndrome
The post-enteritis syndrome was defined earlier (see page 260). This developed in 50 children in Gribbin's study. Why did this syndrome occur? In some patients (14 out of 25 biopsied) small intestinal biopsy indicated that there was a mucosal abnormality, i.e. a post-enteritis enteropathy (Figure. 6.28 ). In the remainder the small intestinal mucosa was normal, although a biopsy in these circumstances could miss a patchy lesion. This, however, was less likely when the double port capsule was used (see page 32). The fact that there is an important difference between those with and without enteropathy was shown by Gribbin et al. (1976), who found that those with an enteropathy were in-patients for 3 weeks to 7 months, only 2 for less than 6 weeks, whereas those without an enteropathy were in-patients for 2 days to 10 weeks (a mean of 3 weeks). Thus the presence of an enteropathy was clearly associated with a more severe illness.
Figure 6.28.
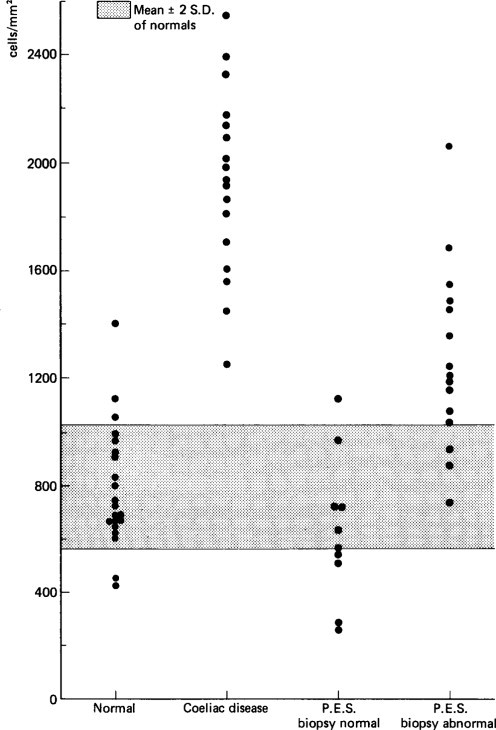
IgA-containing plasma cells in the lamina propria of small intestinal mucosa of children with normal histology, coeliac disease, post-enteritis syndrome (PES) with normal mucosa and post-enteritis syndrome with abnormal mucosa.
(Reproduced by kind permission of Kilby.)
A similar conclusion was reached by Zoppi, Ganello and Gabarro (1977) who found 19 out of 48 children with the post-enteritis syndrome had an enteropathy upon small intestinal biopsy.
Post-enteritis enteropathy
Post-enteritis enteropathy may be defined as persistence of a small intestinal mucosal damage, i.e. enteropathy beyond the natural history of an acute gastrointestinal infection. In practice this is taken to be more than 2 weeks after the onset of acute diarrhoea in most cases.
Aetiology
The cause of post-enteritis enteropathy is uncertain but one explanation is that there is an allergic or hypersensitivity reaction, i.e. there may be an immunological explanation. Another cause may be that there is persistence of gastrointestinal infection beyond the normal span of self-limiting disease or acquisition of further infectious agents causing persistence of the small intestinal mucosal damage. Put another way post-enteritis enteropathy may be due to the following factors:
-
•
Persistent infection with the original infectious agent.
-
•
Reinfection with another pathogen or pathogens.
-
•
Sensitization of food antigens causing persistence of small intestinal mucosal damage.
Post-enteritis-enteropathy: infective enteropathy
Post-enteritis enteropathy may be due to persistent infection i.e. infection continuing to cause damage. The best example of this is provided by infection with enteroadherent E. coli of the cytotoxic variety producing small intestinal mucosal damage (Figure. 6.25). This may be very severe as seen as one variant of the intractable diarrhoea syndrome seen at Queen Elizabeth Hospital and described as Traveller's Diarrhoea with a Vengeance’ (Hutchins et al. 1982). This concerns children known to be well in the land of their birth (United Kingdom) but who develop severe diarrhoea when taken to their ancestral homeland (Indian subcontinent etc.) where they are exposed to a developing community where they are in contact with a heavily contaminated environment. Small intestinal biopsy in these children shows an enteropathy with clear evidence of infection e.g. neutrophils etc. and bacteria may be identified upon the surface of the mucosa adhering to the enterocytes in histological sections. However, the electron microscope gives the most revealing picture. These children have intractable diarrhoea and the damage has persisted long beyond the natural history of the acute infection.
A similar situation may occur in combined immunodeficiency syndromes where for example rotavirus infection may persist with an enteropathy. Chronic rotavirus infection is always associated with disturbed immune function.
The finding of enteropathy in a child with the post-enteritis syndrome may also be due to secondary or acquired infection. For example, Phillips has found astrovirus and adenovirus in the mucosa of children being biopsied for the post-enteritis syndrome who presumably have had intercurrent illnesses accounting for the virus in the abnormal mucosa.
Post-enteritis-enteropathy: food sensitive enteropathy
It is clear that some children with a post-enteritis enteropathy have a food sensitive enteropathy, most often cows' milk sensitive enteropathy. The question that must arise is why does this occur or even why don't all children who develop acute gastroenteritis develop this problem?
A partial explanation may be because there is an immunodeficiency state in those who do either locally in the small intestine or systemically.
In 8 of 20 cases of cows' milk sensitive enteropathy complicating acute gastroenteritis described by Harrison et al. (1976) at the time of diagnosis serum IgA levels were low rising to normal levels with recovery. Haidas et al. in a pilot study found that IgA numbers in the small intestinal mucosa were reduced in children diagnosed as milk-elimination responsive enteropathy, largely post-enteritis using a horseradish peroxidase method. Surprisingly, using an immunofluorescent technique Kilby et al. (1976) found IgA cell numbers to be increased. Kilby's children were more heterogeneous but it is not easy to resolve this difference. Nevertheless the possibility that there is a temporary IgA deficiency as suggested by Soothill (1976) still remains. A temporary opsonization defect has also been described. In addition immaturity of suppressor T-cell function has been suggested. The relative importance of all these immunodeficiency states must still be regarded as uncertain. Another partial explanation may relate to the immaturity of the small intestinal mucosa. The thinness of the mucosa in infants with cows' milk sensitive enteropathy as described by Maluenda et al. (1984) is in accord with this as well as the apparent limited ability of the crypt cells to respond to enterocyte loss. This immaturity may be important too in the ability of food antigens to adhere more readily to the microvillous membrane in immature animals. It is certainly true that post-enteritis food-sensitive enteropathy occurs particularly in young infants and is confined to infants less than 2 years of age.
Increased antigen entry may also play a part. There is certainly evidence that anatomical pathways do exist for antigen entry in the biopsies of children with post-enteritis enteropathy (Jackson et al., 1983). These may be shown by means of horseradish peroxidase uptake in biopsies from children with post-enteritis enteropathy (see Figure. 6.26). Thus there is morphological evidence that antigen entry can occur but what is critical is whether such entry is associated with sensitization. A key factor here may be the antigenicity or allergenicity of the food usually cows' milk fed at the time of acute gastroenteritis. There are both animal and clinical observations to suggest that allergenicity is of critical importance. Figure. 5.3 illustrates an hypothesis linking these factors. The declining importance of cows' milk sensitive enteropathy in Western societies may relate to reduced antigenicity of modern feeds.
Such a food-sensitive enteropathy may overlap with an infective enteropathy as they may both coexist. Walker-Smith et al. (1978) described 5 children with post-enteritis enteropathy who responded to a cows' milk-free diet yet who also had evidence of infection at the time of biopsy.
Malnutrition may play a role by interfering with the ability of the damaged mucosa to recover; however, the author believes that the importance of malnutrition as a cause of enteropathy has been exaggerated in the past (see Chapter. 13).
Diagnosis
When an infant with the post-enteritis syndrome has an abnormal mucosa the nature of the lesion as described above will influence diagnosis. When bacteria are present with the typical morphology of enteroadherent E. coli then the diagnosis is clear (see Figure. 6.25). If a child being fed cows' milk has an enteropathy with the features described in Chapter. 5 then cows' milk sensitive enteropathy is likely. If he then responds rapidly to a cows' milk-free diet with relief of diarrhoea and weight gain this is the likely diagnosis. Management is discussed in Chapter. 5.
Post-enteritis syndrome without an enteropathy
Turning attention now to the mechanism of chronic diarrhoea in children with this syndrome who do not have an enteropathy, the role of disturbed immunological function must also be considered. Avigad et al. (1978) have investigated the flora of the small intestine in children with the post-enteritis syndrome. They cultured duodenal juice and the homogenized duodenal mucosa from children with the post-enteritis syndrome, children with other causes of chronic diarrhoea, and a control group of children without diarrhoea. They found no significant difference in bacterial flora as measured by total bacterial flora within these three groups. The role of bacterial colonization of the small intestine and enteric disease was discussed briefly in Chapter. 1. Its role in this syndrome is not yet clear but Avigad et al. (1978) using an immunofluorescent technique were able to demonstrate mucosal antibodies against the bacteria grown from the luminal fluid or mucosa of the same children from children with the post-enteritis syndrome, and other causes of chronic diarrhoea. The finding of such antibodies was unrelated to the presence or absence of an enteropathy. Mucosal antibody to these non-pathogenic bacteria, obtained from the duodenal juice or homogenate culture, was present in 8 of 9 children with the post-enteritis syndrome regardless of whether an enteropathy was present. Antibody was also found in three children with other causes of chronic diarrhoea, but antibody was not present in the control group who did not have diarrhoea at the time of biopsy, except in one child who had previously had an enteropathy, and possibly one doubtful case who also had a previous enteropathy. The role of such small intestinal mucosal antibodies against non-pathogenic organisms cultured from the small intestine of these children is not clear in relation to the pathogenesis of chronic diarrhoea in children, but clearly warrants further study.
Phillips et al. (1978) have used the scanning electron microscope to study the surface of small intestinal lymphoid follicles obtained on proximal small intestinal biopsy which was morphologically normal (Figure. 6.29 ). Normally, no bacteria adhere to the surface of such follicles but as Figure. 6.29 shows there can be adhesion of rod-like bacteria to the surface, whereas bacteria were not adhering to the rest of the biopsy. This was a specimen taken from a child with post-enteritis syndrome shown to be producing antibody to rod-like bacteria. This preferential adhesion of bacteria to the surface of the lymphoid follicular region is interesting because there is now good evidence that antigen entry occurs through the epithelium overlying lymphoid follicles, and that immunoblast cells are primed at these sites prior to migration to the lamina propria of the gut mucosa where they secrete immunoglobulin (see Chapter. 1). Thus adherence of bacteria to the overlying epithelium of the lymph follicle may be related to antibody production and may have significance in the pathogenesis of this child's continuing diarrhoea.
Figure 6.29.
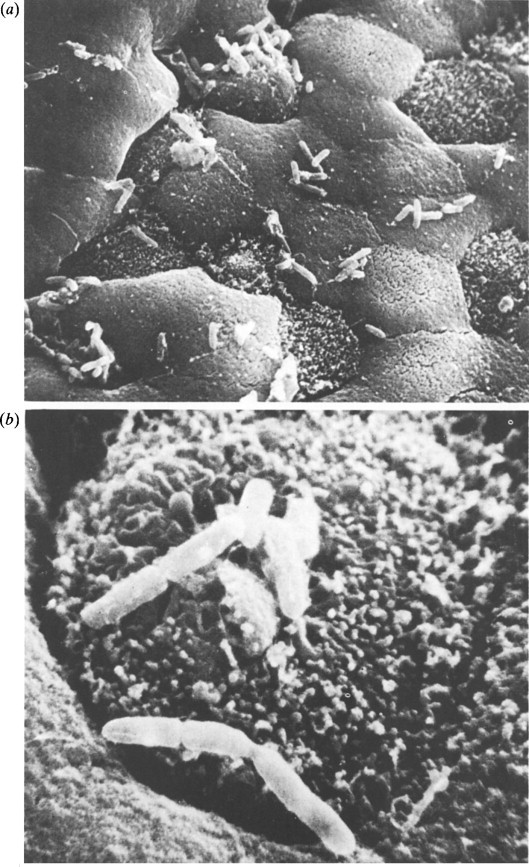
(a) Scanning electron micrograph of lymphoid follicle in a small intestinal biopsy sample from a child with chronic diarrhoea demonstrating rods adherent to the surface of the mucosa. × 3300.(b) Surface of a single cell, × 14 000.
(Reproduced by kind permission of Phillips.) (Reproduced by kind permission of Phillips.)
Clearly, a great deal more research is required into the genesis of post-enteritis diarrhoea. This is an important subject both in the developed and developing communities, but in the latter it is a problem of enormous size.
References
- ADLER J.L., ZICKL R. Winter vomiting disease. Journal of Infectious Diseases. 1969;119:668–673. doi: 10.1093/infdis/119.6.668. [DOI] [PubMed] [Google Scholar]
- ANDERSON K.J., MCLAUGHLAN P., DEVEY M.E., COOMBS R.R.A. Anaphylactic sensitivity of guinea-pigs drinking different preparations of cows’ milk and infant formulae. Clinical and Experimental Immunology. 1979;35:454–461. [PMC free article] [PubMed] [Google Scholar]
- ARAYA M., SILINK S.J., NOBILE S., WALKER-SMITH J.A. Blood vitamin levels in children with gastroenteritis. Australian and New Zealand Journal of Medicine. 1975;5:239. doi: 10.1111/j.1445-5994.1975.tb04576.x. [DOI] [PubMed] [Google Scholar]
- ARMISTEAD, J., KELLY, D. and WALKER-SMITH, J. A., (1987). Evaluation of infant feeding in acute gastroenteritis. (In press) [DOI] [PubMed]
- ARNEIL G.C., CHIN K.C. Lower solute milks and reduction of hypernatraemia in young Glasgow infants. Lancet. 1979;ii:840. doi: 10.1016/s0140-6736(79)92187-1. [DOI] [PubMed] [Google Scholar]
- ASHKENAZI S., DANZIGER Y., VARSANO Y., PEILAN J., MIMOUNI M. Treatment of Campylobacter gastroenteritis. Archives of Disease in Childhood. 1987;62:84–85. doi: 10.1136/adc.62.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVIGAD S., MANUEL P., BAMPOE V., WALKER-SMITH J.A., SHINER M. Small intestinal mucosal antibodies against antigens of non-pathogenic luminal or mucosal bacteria in young children with and without diarrhoea. Lancet. 1978;i:1130. doi: 10.1016/s0140-6736(78)90304-5. [DOI] [PubMed] [Google Scholar]
- AXTON J. Diarrhoea and Malnutrition in Childhood. Butterworths; London: 1986. Gastrointestinal structure and function in measles; pp. 166–171. [Google Scholar]
- BARNES, G. L. (1973). Studies in Infantile Gastroenteritis, MD Thesis, Department of Paediatrics, University of Otago, Dunedin, New Zealand.
- BARNES G.L., TOWNLEY R.R.W. Duodenal mucosal damage in 31 infants with gastroenteritis. Archives of Disease in Childhood. 1973;48:343. doi: 10.1136/adc.48.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEADS G.M., HALL C., GREEN J., FLEWETT T.H., LAMOULIATTE F., PASQUIER F. An enveloped virus in stools of children and adults with gastroenteritis that resembles the Breda virus of calves. Lancet. 1984;i:1050–1052. doi: 10.1016/S0140-6736(84)91454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIDDULPH J., PANGKATANA P. Weaning diarrhoea. Papua and New Guinea Medical Journal. 1971;14:1. [PubMed] [Google Scholar]
- BILLINGHAM J.D. Campylobacter enteritis in The Gambia. Transcriptions of The Royal Society for Tropical Medicine and Hygiene. 1881;75:641–644. doi: 10.1016/0035-9203(81)90140-1. [DOI] [PubMed] [Google Scholar]
- BISHOP R.F., DAVIDSON G.P., HOLMES I.H., RUCK B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;ii:1281. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- BISHOP R.F., DAVIDSON G.P., NOLMES I.H., RUCK B.J. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974;i:149. doi: 10.1016/s0140-6736(74)92440-4. [DOI] [PubMed] [Google Scholar]
- BISHOP, R.F., CAMERON, D. J. S., BARNES, G. P., HOLMES, I. H. and RUCK, B. J., (1976). The Aetiology of Diarrhoea in Newborn Infants. Ciba Foundation Symposium, 42 (new series) Acute Diarrhoea in Childhood. Elsevier Excerpta Medica North-Holland [DOI] [PubMed]
- BLACK R.E., MERSON M.H., RAHMAN A.S. A two-year study of bacterial, viral and parasitic agents associated with diarrhoea in rural Bangladesh. Journal of Infectious Diseases. 1980;142:660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKLOW N.R., DOLIN R., FEDSON D.S., DUPONT H., NORTHRUP R.S., HORNICK R.B., CHANOCK R.M. Acute infectious non-bacterial gastroenteritis: aetiology and pathogenesis. Annals of Internal Medicine. 1972;76:993. [Google Scholar]
- BLASER M.J., CRAVENS J., POWERS B.W., LAFORCE F.M., WANG W.-LL. Campylobacter enteritis associated with unpasteurised milk. American Journal of Medicine. 1979;67:715–718. doi: 10.1016/0002-9343(79)90272-9. [DOI] [PubMed] [Google Scholar]
- BOEDEKER E.C., SHERMAN P.M. Mechanisms of Escherichia coli enteritis. Front. 1986;13:307–330. [Google Scholar]
- BORTULUSSI R., SZYMANSKI M., HAMILTON R. Studies on the etiology of acute infantile diarrhoea. Pediatric Research. 1974;8:379. [Google Scholar]
- BRAUN O.H. Bray's discovery of pathogenic E. coli as a cause of infantile gastroenteritis. Archives of Disease in Childhood. 1974;49:668. doi: 10.1136/adc.49.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY J. Isolation of antigenically homogenous strains of Bacterium coli neapolitum from summer diarrhoea of infants. Journal of Pathology and Bacteriology. 1945;57:239. [Google Scholar]
- BRYDEN A.S., THOULESS M.E., HALL C.J., FLEWETT T.H., WHARTON B.A., MATTHEW P.M., CRAIG I. Rotavirus infection in a special care baby unit. Journal of Infection. 1982;4:43–48. doi: 10.1016/s0163-4453(82)90988-4. [DOI] [PubMed] [Google Scholar]
- BACHINO J.J., SUCHY F.J., SNYDER J.W. Bacterial diarrhoea in infants and children. Perspectives in Pediatric Pathology. 1984;8:163–166. [PubMed] [Google Scholar]
- BULLEN C.L., WILLIS A.T. Resistance of the breast fed infant to gastroenteritis. British Medical Journal. 1971;iii:338. doi: 10.1136/bmj.3.5770.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE V., KERRY K.R., ANDERSON C.M. The relationship of dietary lactose to refractory diarrhoea in infancy. Australian Paediatric Journal. 1965;I:147. [Google Scholar]
- BURKE V., GRACEY M., MASTERS P. Rotavirus in children. Journal of Infectious Diseases. 1985;152:646. doi: 10.1093/infdis/152.3.646. [DOI] [PubMed] [Google Scholar]
- CARLSON J.A., MIDDLETON P.J., SZYMANSKI M.T., HUBER J., PETRIC M. Fatal rotavirus gastroenteritis an analysis of 21 cases. American Journal of Diseases in Children. 1978;132:477–479. doi: 10.1001/archpedi.1978.02120300037006. [DOI] [PubMed] [Google Scholar]
- CARR M.E., DONALD G., MCKENDRICK W., SPYRIDAKIS T. The clinical features of infantile gastroenteritis due to rotavirus. Scandinavica Journal of Infectious Disease. 1976;8:241. doi: 10.3109/inf.1976.8.issue-4.04. [DOI] [PubMed] [Google Scholar]
- CAUL E.O., PAVER W.K., CLARKE S.K.R. Coronavirus particules in faeces from patients with gastroenteritis. Lancet. 1975;i:1192. doi: 10.1016/S0140-6736(75)93176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUL E.O., APPLETON H. The electron microscopical and physical characteristics of small round human fecal viruses: an interim scheme for classification. Journal of Medical Virology. 1982;9:257–265. doi: 10.1002/jmv.1890090403. [DOI] [PubMed] [Google Scholar]
- CHAMPSAUR H., QUESTIZUX E., PREVOT J., HENRY-AMAR M., GOLDSZMIDT D., BOURJOUANE M., BACH C. Rotavirus carriage, asymptomatic infection, and disease in the first two years of life. I. Virus shedding. Journal of Infectious Diseases. 1984;149:667–674. doi: 10.1093/infdis/149.5.667. [DOI] [PubMed] [Google Scholar]
- CHANDRA, R. K. (1976). Immunological consequences of malnutrition including fetal growth retardation. In Food and Immunology. Swedish Nutrition Foundation Symposium XIII. Almqvist and Eiksell International, Stockholm
- CHANTEMESSE A., WIDAL F. Sur les microbes de la dysenteric epidemique. Bulletin de l'Academic de medicine (Paris) 1888;19:522. [Google Scholar]
- CHERNESKY M., CASTRICIANO S., MAHONY J., DELONG D. Examination of the Rotazyme II enzyme immunoassay for the diagnosis of rotavirus gastroenteritis. Journal of Clinical Microbiology. 1985;22:462–464. doi: 10.1128/jcm.22.3.462-464.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBA S., NAKATA S., NAKAMURA I., TANIGUCHI K., URASAWA S., FUJINAGA K., NAKAO T. Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet. 1983;ii:954–957. doi: 10.1016/s0140-6736(83)90463-4. [DOI] [PubMed] [Google Scholar]
- CHRYSTIE I.L., TOTTERDELL B., BAKER M.J., SCOPES J.W., BANATVALA J.E. Rotavirus infections in a maternity unit. Lancet. 1975;ii:79. doi: 10.1016/s0140-6736(75)90525-5. [DOI] [PubMed] [Google Scholar]
- CHUNG A.W., VISCOROVA B. The effect of early oral feeding versus early oral starvation on the course of infantile gastroenteritis. Journal of Pediatrics. 1948;33:14–22. doi: 10.1016/s0022-3476(48)80148-4. [DOI] [PubMed] [Google Scholar]
- CLARKE S.K.R., CAUL E.O., EGGLESTONE S.I. The human enteric corona viruses. Postgraduate Medical Journal. 1979;55:135–141. doi: 10.1136/pgmj.55.640.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUSEN C.R., CHRISTIE D.L. Chronic diarrhoea in infants caused by adherent enteropathogenic Escherichia coli. Journal of Pediatrics. 1982;100:358–361. doi: 10.1016/s0022-3476(82)80429-0. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE A., MAHALANABIS D., JALAN K.N., MAITRA T.K., AGARWAL S.K., BAGCHI D.K., INDRA S. Evaluation of a sucrose/electrolyte solution for oral rehydration in acute infantile diarrhoea. Lancet. 1977;i:1333. doi: 10.1016/s0140-6736(77)92550-8. [DOI] [PubMed] [Google Scholar]
- COIRO I.R.R., BENDATI M.M.A., NETO A.J.A., HEUSER M.C.I., VASCONCELLOS V.L. Rotavirus infection in Brazilian children with acute enteritis: a seasonal variation study. American Journal of Tropical Medicine and Hygiene. 1983;32:1186–1189. doi: 10.4269/ajtmh.1983.32.1186. [DOI] [PubMed] [Google Scholar]
- COLLE E., AROUB E., RAILE R. Hypertonic dehydration (hypernatraemia): the role of feedings high in solute. Pediatrics. 1958;2:5. [PubMed] [Google Scholar]
- COTTERILL A.M., WALKER-SMITH J.A. Childhood epidemiology: gastro-intestinal tract. British Medical Bulletin. 1986;42:176–180. doi: 10.1093/oxfordjournals.bmb.a072118. [DOI] [PubMed] [Google Scholar]
- CREAMER B., LEPPARD P. Post-mortem examination of a small intestine in the coeliac syndrome. Gut. 1965;6:466. doi: 10.1136/gut.6.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUBITT W.D., BARRETT A.D.T. Propagation of human candidate calicivirus in cell culture. Journal of General Virology. 1984;65:1123–1126. doi: 10.1099/0022-1317-65-6-1123. [DOI] [PubMed] [Google Scholar]
- CUBBITT W.D., BARRETT A.D. Propagation and preliminary characterization of a chicken candidate calicivirus. Journal of General Virology. 1985;66:1431–1438. doi: 10.1099/0022-1317-66-7-1431. [DOI] [PubMed] [Google Scholar]
- CUBBITT W.D., MCSWIGGAN D.A., MOORE W. Winter vomiting disease caused by calicivirus. Journal of Clinical Pathology. 1979;32:786–793. doi: 10.1136/jcp.32.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRY J. Infecting dose of salmonella. Lancet. 1976;i:1296. doi: 10.1016/s0140-6736(76)91765-7. [DOI] [PubMed] [Google Scholar]
- CUTTING W. Oral treatment of diarrhoea in children. Journal of Maternal and Child Health. 1979;4:276–281. [Google Scholar]
- DARROW D.C., PRATT E.L., FLETT J., GAWBLE A.H., WIESE H.F. Disturbances of water and electrolytes in infantile diarrhoea. Pediatrics. 1949;5:129. [PubMed] [Google Scholar]
- DAVIDSON G.P., BISHOP R.F., TOWNLEY R.R.W., HOLMES I.H., RUCK B.J. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975;i:242. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G.P., GOLLER I., BISHOP R.F., TOWNLEY R.R.W., HOLMES I.H., RUCK B.J. Immunofluorescence in duodenal mucosa of children with acute enteritis due to a new virus. Journal of Clinical Pathology. 1975;28:263. doi: 10.1136/jcp.28.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES D.P., ANSARI B.M., MANDAL B.K. Hypernatraemia and gastroenteritis. Lancet. 1977;i:252. doi: 10.1016/s0140-6736(77)91042-x. [DOI] [PubMed] [Google Scholar]
- DEKEYSER P., GOSSUIN-DETRAIN M., BUTZL J.P., STERNON J. Acute enteritis due to related vibrio: first positive stool cultures. Journal of Infectious Diseases. 1972;125:390–392. doi: 10.1093/infdis/125.4.390. [DOI] [PubMed] [Google Scholar]
- DE PEYER E., WALKER-SMITH J.A. Cow's milk intolerance presenting as necrotizing enterocolitis. Helvetica Paediatrica Acta. 1977;32:509. [PubMed] [Google Scholar]
- DIGEON B., WALKER-SMITH J.A. Food intolerance and gastrointestinal disease in infancy: Personal Practice. Digestive Diseases. 1986;4:139–147. doi: 10.1159/000171144. [DOI] [PubMed] [Google Scholar]
- DIXON J. Effect of antibiotic treatment on the duration of excretion of Salmonella typhimurium by children. British Medical Journal. 1965;ii:1343. doi: 10.1136/bmj.2.5474.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN R., ROESSNER K.D., TREANOR J.J., REICHMAN R.C., PHILIPS M., MADORE H.P. Radioimmunoassay for detection of the Snow Mountain agent of viral gastroenteritis. Journal of Medical Virology. 1986;19:11–18. doi: 10.1002/jmv.1890190103. [DOI] [PubMed] [Google Scholar]
- DOLIN R., BLACKLOW N.R., DUPONT H., FORMAL S.B., BUSCHO R.F., KASEL J.A., CHAMES R.P., HORNICK R., CHANOCK R.M. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. Journal of Infectious Diseases. 1971;123:307–312. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- DOLIN R., TREANOR J.J., MADORE M.P. Novel agents of viral enteritis in humans. Journal of Infectious Diseases. 1987;155:365–376. doi: 10.1093/infdis/155.3.365. [DOI] [PubMed] [Google Scholar]
- DORMAN, D. (1968). Personal communication
- DORMAN, D. (1972). Personal communication
- DUGDALE A., LORRELL S., GIBBS V. Re-feeding after acute gastroenteritis. A controlled study. Archives of Diseases of Childhood. 1982;57:76–78. [PMC free article] [PubMed] [Google Scholar]
- DUPONT H.L., FORMAL S.B., HORNICK R.B., SNYDER M.J., LIBONATI J.P., SHEAHAN D.P., LABREC E.H., KALPAS J.P. Pathogenesis of Escherichia coli diarrhoea. New England Journal of Medicine. 1971;285:1. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- EIDEN J., VONDERFECHT S., YOLKEN R.H. Evidence that a novel rotavirus-like agent of rats can cause gastroenteritis in man. Lancet. 1986;ii:8–10. doi: 10.1016/s0140-6736(85)90057-1. [DOI] [PubMed] [Google Scholar]
- ELLIOTT E.J., WALKER-SMITH J.A. Salmonellosis. The Practitioner. 1987 AAAIn press) [PubMed] [Google Scholar]
- EVANS N.A.P., HENDRICKSE R.G., MACFARLANE S.B.J., MEIRING H.E., MILLA P.J., MOODY J.B., SANDHU B.K., SMITH M.L., TAYLOR C.J., WALKER-SMITH J.A. Loperamide in acute diarrhoea in childhood results of a double blind, placebo controlled multicentre clinical trial. British Medical Journal. 1984;289:1263–1267. doi: 10.1136/bmj.289.6454.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN R.A., KAMATH K.R., RAO P.S.S., WEBB J.K.G. Infection and disease in a group of south Indian families. American Journal of Epidemiology. 1969;289:364. doi: 10.1093/oxfordjournals.aje.a120950. [DOI] [PubMed] [Google Scholar]
- FELDMAN R.A., BHAT P., KAMATH K.R. Infection and disease in a group of south Indian families. IV. Bacteriologic methods and a report of the frequency of enteric bacterial infections in preschool children. American Journal of Epidemiology. 1970;92:367. doi: 10.1093/oxfordjournals.aje.a121218. [DOI] [PubMed] [Google Scholar]
- FENNER F. Infectious disease and social change. Medical Journal of Australia. 1971;i:1043. doi: 10.5694/j.1326-5377.1971.tb88037.x. [DOI] [PubMed] [Google Scholar]
- FERGUSON A., SNODGRASS D.P. Intestinal architecture and epithelial cell kinetics during and after experimental rotavirus infection in lambs. Acta Paediatrica Belgica. 1978;31:109. [Google Scholar]
- FERGUSON A., MCCLURE J.P., TOWNLEY R.R.W. Intraepithelial lymphocyte counts in small intestinal biopsies from children with diarrhoea. Acta Paediatrica Scandinavica. 1976;65:541. doi: 10.1111/j.1651-2227.1976.tb04929.x. [DOI] [PubMed] [Google Scholar]
- FIELD M. Intestinal secretion: effect of cyclic AMP and its role in cholera. New England Journal of Medicine. 1971;2854:1137. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- FIELD, M. (1986). Effect of enterotoxins. Presented at The Joint Meeting of North American Society for Pediatric Gastroenterology and European Society for Paediatric Gastroenterology and Nutrition
- FINBERG L.F. The management of the critically ill child with dehydration secondary to diarrhoea. Pediatrics. 1970;45:1029. [PubMed] [Google Scholar]
- FINBERG L.F. Hypernatraemic (hypertonic) dehydration in infants. New England Journal of Medicine. 1973;289:196. doi: 10.1056/NEJM197307262890407. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN R.A., SCIORTINO C.V., MCINTOSH M.A. Role of iron in microbe-host interactions. Review of Infectious Diseases. 1983;5:S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- FLEWETT T.H. Diagnosis of enteritis virus. Proceedings of the Royal Society of Medicine. 1976;69:693. [PMC free article] [PubMed] [Google Scholar]
- FLEWETT, T. H. (1976b). Implications of Recent Virological Researches. Ciba Foundation Symposium 42 (new series) Acute Diarrhoea in Childhood. Elsevier Excerpta Medica North-Holland [DOI] [PubMed]
- FLEWETT T.H. Rotavirus vaccines—achievements and prospects. Archives of Disease in Childhood. 1986;61:211–212. doi: 10.1136/adc.61.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEWETT T.H., DAVIES H. Caliciviruses in man. Lancet. 1976;i:311. doi: 10.1016/s0140-6736(76)91450-1. [DOI] [PubMed] [Google Scholar]
- FLEWETT T.H., BRYDEN A.S., DAVIES H. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974;ii:66–67. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- FLEWETT T.H., BRYDEN A.S., DAVIES H. Virus particles in gastroenteritis. Lancet. 1973;ii:1497. doi: 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- FLORES J., PEREZ-SCHAEL I., BOEGGEMAN E., WHITE L., PEREZ M., PURCELL R., HOSHINO Y., MIDTHUN K., CHANOCK R.M., KAPIKIAN A.Z. Genetic relatedness among human rotaviruses. Journal of Medical Virology. 1985;17:135–143. doi: 10.1002/jmv.1890170206. [DOI] [PubMed] [Google Scholar]
- FLORES J., PEREZ-SCHAEL I., GONZALEZ M., GARCIA D., PEREZ M., DAOUD N., CUNTO W., CHANOCK R.M., KAPIKIAN A.Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987;i:882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- FOBERG U., FRYDEN A., KIHLSTROM E. Yersinia Enterocolitica Septicaemia: Clinical and Microbiologic Aspects. Scandinavian Journal of Infectious Diseases. 1986;18:269–279. doi: 10.3109/00365548609032337. [DOI] [PubMed] [Google Scholar]
- FONTEYNE J., ZUSIS G., LAMBERT J.P. Recurrent rotavirus gastroenteritis. Lancet. 1978;i:983. doi: 10.1016/s0140-6736(78)90263-5. [DOI] [PubMed] [Google Scholar]
- FORD R.P.K., MENZIES I.S., PHILLIPS A.D., WALKER-SMITH J.A., TURNER M.W. Intestinal sugar permeability: relationship to diarrhoeal disease and small bowel morphology. Journal of Pediatric Gastroenterology and Nutrition. 1985;4:568–575. [PubMed] [Google Scholar]
- FORMAL, S.B., GEMSKI, P., GIANELLA, R. A. and TAKEUCHI, A., (1976). Studies on The Pathogenesis of Enteric Infections Caused by Invasive Bacteria. Acute diarrhoea in childhood. Ciba Foundation Symposium 42, new series. Elsevier, Excerpta Medica, North Holland, p. 27 [DOI] [PubMed]
- FRANCIS D. Diets for Sick Children. Blackwell Scientific Publications; London: 1987. pp. 77–128. [Google Scholar]
- GALBRAITH N.S. Surveillance of foodborn infections in England and Wales. Chemistry and Industry. 1985;5:148–152. [Google Scholar]
- GAMBLE, J. L., Early history of fluid replacement therapy. Pediatrics, II, 554 [PubMed]
- GARROW J.S., SMITH R., WARD E.E. Electrolyte Metabolism in Severe Infantile Malnutrition. Pergamon Press; London: 1968. [Google Scholar]
- GIANELLA R.A., GOTS R.E., CHARNEX A.N., GREENOUGH B., FORMAL S.B. Pathogenesis of salmonella-mediated fluid secretion. Alteration of adenylate cyclase and inhibition by indomethacin. Gastroenterology. 1975;69:1238. [PubMed] [Google Scholar]
- GILES C., SANGSTER G., SMITH J. Epidemic gastroenteritis in infants in Aberdeen during 1947. Archives of Disease in Childhood. 1949;24:45. doi: 10.1136/adc.24.117.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES R.G., MONIF M.D., HOOD C.I. Ileocolitis associated with measles (Rubella) American Journal of Diseases in Children. 1970;120:245. doi: 10.1001/archpedi.1970.02100080129014. [DOI] [PubMed] [Google Scholar]
- GOULD, S. (1977). MD Thesis
- GRACEY M. Enteric disease in young Australian aborigines. Australian and New Zealand Journal of Medicine. 1973;3:576. doi: 10.1111/j.1445-5994.1973.tb04298.x. [DOI] [PubMed] [Google Scholar]
- GRACEY M., BURKE V., ROBINSON J. Aeromonas-associated gastroenteritis. Lancet. 1982;ii:1304–1306. doi: 10.1016/s0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- GRACEY M., BURKE V., ROBINSON J. Aeromonas spp. in travellers’ diarrhoea. British Medical Journal. 1984;289:658. doi: 10.1136/bmj.289.6446.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG H.B., VALDESUSO J.R., KALICA A.R., WYATT R.G., MCAULIFFE V.J., KAPIKIAN A.Z., CHANOCK R.M. Proteins of Norwalk virus. Journal of Virology. 1981;37:994–999. doi: 10.1128/jvi.37.3.994-999.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBBIN M., WALKER-SMITH J.A., WOOD C.B.S. A twelve month prospective survey of admissions to the gastroenteritis unit of a children's hospital. Acta Paediatrica Belgica. 1976;29:69. [PubMed] [Google Scholar]
- GRIBBIN M., WALKER-SMITH J.A., WOOD C.B.S. Delayed recovery following acute gastroenteritis. Acta Paediatrica Belgica. 1976;29:167. [PubMed] [Google Scholar]
- GRIBBIN M., WALKER-SMITH J.A., WOOD C.B.S. Gastroenteritis and its sequelae: A prospective review of experience in a children's hospital. Acta Paediatrica Scandinavica. 1976;64:145. [Google Scholar]
- GRIBBIN M., WALKER-SMITH J.A., WOOD C.B.S. Delayed recovery following acute gastroenteritis. Acta Paediatrica Belgica. 1976;29:167–171. [PubMed] [Google Scholar]
- GRILLNER L., BROBERGER U., CHRYSTIE I., RANJSO U. Rotavirus infections in newborns: an epidemiological and clinical study. Scandinavian Journal of Infectious Diseases. 1985;17:349–355. doi: 10.3109/13813458509058774. [DOI] [PubMed] [Google Scholar]
- GRIMWOOD K., ABBOTT G.D., FERGUSON D.M., JENNINGS L.C., ALLAN J.M. Spread of rotavirus within families: a community based study. British Medical Journal. 1983;287:575–577. doi: 10.1136/bmj.287.6392.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROOTHUIS J.R., BERMAN S., CHAPMAN J. Effect of carbohydrate ingested on outcome in infants with mild gastroenteritis. Journal of Pediatrics. 1986;25:85–88. doi: 10.1016/s0022-3476(86)80924-6. [DOI] [PubMed] [Google Scholar]
- GRUSKAY F.L., COOK R.E. The gastrointestinal absorption of unaltered protein in normal infants and in infants from diarrhoea. Pediatrics. 1955;16:763. [PubMed] [Google Scholar]
- GRYBOSKI J.D. Gastrointestinal milk allergy in infants. Pediatrics. 1967;40:354–362. [PubMed] [Google Scholar]
- GURWITH M., WENMAN W., HINDE D., FELTHAM S., GREENBERG H. A prospective study of rotavirus infection in infants and young children. Journal of Infectious Diseases. 1981;144:218–244. doi: 10.1093/infdis/144.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON, J.R., GALL, D. G., BUTLER, D. G. and MIDDLETON, P. J., (1976). Viral Gastroenteritis: Recent Progress, Remaining Problems. Ciba Foundation Symposium 42 (new series). Acute Diarrhoea in Childhood. Elsevier Excerpta Medica, North-Holland [DOI] [PubMed]
- HANSEN, J. D. L., (1968). Features and Treatment of Kwashiorkor at the Cape. Calorie Deficiencies and Protein Deficiencies. Proceedings of a colloquium. Churchill, London, p. 33
- HAQUE K., ALFRAY A. Is it necessary to regrade milk after acute gastroenteritis in children? Tropical and Geographical Medicine. 1983;35:369–373. [PubMed] [Google Scholar]
- HARRISON, B.M., KILBY, A., WALKER-SMITH, J. A., FRANCE, N. E. and WOOD, C. B. S., Cow's milk protein intolerance: A possible association with gastroenteritis, lactose intolerance and IgA deficiency. British Medical Journal, ii, 1501 [DOI] [PMC free article] [PubMed]
- HERNESKY M., CASTRICIANO S., MAHONY J., DELONG D. Examination of the Rotazyme II enzyme immunoassay for the diagnosis of rotavirus gastroenteritis. Journal of Clinical Microbiology. 1985;22:462–464. doi: 10.1128/jcm.22.3.462-464.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL L.W., STUART H.C. A soy-bean food preparation for feeding infants with milk allergy. Journal of the American Medical Association. 1929;93:986. [Google Scholar]
- HIRSCHORN N., MCCARTHY B.J., RANNEY B., HIRSCHORN M.A., WOODWARD S.T., LACAPA A., CASH R.A., WOODWARD W.E. Ad libitum oral glucose-electrolyte therapy for acute diarrhoea in Apache children. Journal of Pediatrics. 1973;83:562. doi: 10.1016/s0022-3476(73)80215-x. [DOI] [PubMed] [Google Scholar]
- HJELT K.B., GRAUBALLE P.C., NIELSEN O.H., SCHIOTZ P.O., KRASILNIKOFF P.A. Rotavirus antibodies in the mother and her breast fed infant. Journal of Pediatric Gastroenterology and Nutrition. 1985;4:414–420. doi: 10.1097/00005176-198506000-00016. [DOI] [PubMed] [Google Scholar]
- HOLMES I.H., RUCK B.J., BISHOP R.F. Infantile enteritis viruses: morphogenesis and morphology. Journal of Virology. 1975;16:937. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES I.H., RODGER S.M., SCHNAGL R.P., RUCK B.J., GUST I.D., BISHOP R.F., BARNES G.L. Is lactase the receptor and uncoating enzyme for infantile enteritis (rota) viruses? Lancet. 1976;i:1387. doi: 10.1016/s0140-6736(76)93032-4. [DOI] [PubMed] [Google Scholar]
- HUTCHINS P., MATTHEWS T.H.J., MANLY J.A.E., LAWRIE B., WALKER-SMITH J.A. Comparison of oral sucrose and glucose electrolyte solutions in the out-patient management of acute gastroenteritis in infancy. Journal of Hygiene. 1979;85:15. doi: 10.1017/s0022172400025420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHINS P., WILSON C., MANLY J.A.E., WALKER-SMITH J.A. Oral solutions for infantile gastroenteritis—variations in composition. Archives of Disease in Childhood. 1980;55:616. doi: 10.1136/adc.55.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHINS P., HINDOCHA P., PHILLIPS A., WALKER-SMITH J.A. Traveller's diarrhoea with a vengence in children of UK immigrants visiting their parental homeland. Archives of Disease in Childhood. 1982;57:208–211. doi: 10.1136/adc.57.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRONSIDE A.G. Gastroenteritis in infancy. British Medical Journal. 1973;i:284. doi: 10.1136/bmj.1.5848.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRONSIDE A.G., TUXFORD A.F., HEYWORTH B. A survey of infantile gastroenteritis. British Medical Journal. 1970;iii:20. doi: 10.1136/bmj.3.5713.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISOLAURI E., VESIKARI T., SAHA P., VIANDER M. Milk versus no milk in rapid refeeding after acute gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition. 1986;5:254–261. [PubMed] [Google Scholar]
- JACKSON D., WALKER-SMITH J.A., PHILLIPS A.D. Macromolecular absorption by histologically normal and abnormal small intestine mucosa in childhood: An in vitro study using organ culture. Journal of Pediatric Gastroenterology and Nutrition. 1983;2:235–248. [PubMed] [Google Scholar]
- JELLIFFE D.B., JELLIFFE E.F. The weanling's dilemma. Lancet. 1978;i:611. doi: 10.1016/s0140-6736(78)91058-9. [DOI] [PubMed] [Google Scholar]
- JENKINS H.R., PINCOT J.R., SOOTHILL J.F., MILLA P.J., HARRIES J.T. Food allergy, the major cause of infantile colitis. Archives of Disease in Childhood. 1984;59:326–329. doi: 10.1136/adc.59.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON M.E., UHNOO I., SVENSSON L., PETTERSSON C.A., WADELL G. Enzyme-linked assay for detection of enteric adenovirus 41. Journal of Medical Virology. 1985;17:19–27. doi: 10.1002/jmv.1890170104. [DOI] [PubMed] [Google Scholar]
- JULKUNEN I., SAVOLAINED J., HAUTANEN A., HOVE T. Detection of rotavirus in faecal specimens by enzyme immunoassay, latex agglutination and electron microscopy. Scandinavian Journal of Infectious Diseases. 1985;17:245–249. doi: 10.3109/inf.1985.17.issue-3.02. [DOI] [PubMed] [Google Scholar]
- KALKAY M.N., AYANIAN Z.S., LEHAF E.A., BALDI A. Campylobacter-induced toxic megacolon. American Journal of Gastroenterology. 1983;78:557–559. [PubMed] [Google Scholar]
- KAPIKIAN A.Z., WYATT R.G., DOLIN R., THORNHILL T.S., KALICA A.R., CHANOCK R.M. Visualization by immune electron microscopy of a 27 nm particle associated with acute infectious nonbacterial gastroenteritis. Journal of Virology. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPIKIAN A.Z., CLINE W.L., MEBUS C.A. New complement-fixation test for the human reovirus-like agent of infantile gastroenteritis. Lancet. 1975;i:1956. doi: 10.1016/s0140-6736(75)91827-9. [DOI] [PubMed] [Google Scholar]
- KAPIKIAN A.Z., KIM H.W., WYATT R.G., CLINE W.L., ARROBIO P., BRANDT C.D., RODRIQUEZ W.J., SACK D.A., CHANOCK R.M., PARROTT R.H. Human reovirus-like agent as the major pathogen associated with ‘winter’ gastroenteritis in hospitalized infants and young children. New England Journal of Medicine. 1976;294:965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- KARMALI M.A., FLEMING P.C. Campylobacter enteritis in children. Journal of Pediatrics. 1979;94:527–533. doi: 10.1016/s0022-3476(79)80004-9. [DOI] [PubMed] [Google Scholar]
- KATTAMIS C., SYRIOPOULOU V., HADJIMINAS J., TSIVITANIDOU-KAKOUROU T. Effectiveness of intravenous Augmentin in the treatment of thalassaemic patients with Yersinia enterocolitica infections. Journal of Antimicrobial Chemotherapy. 1984;14:303–305. doi: 10.1093/jac/14.3.303. [DOI] [PubMed] [Google Scholar]
- KELLY D.A., PRICE E., JAM B., WRIGHT V., ROSSITER M., WALKER-SMITH J.A. Yersinia enterocolitis in iron overload. Journal of Pediatric Gastroenterology and Nutrition. 1987;6:643–646. doi: 10.1097/00005176-198707000-00027. [DOI] [PubMed] [Google Scholar]
- KIDD A.H., HARLEY E.H., ERASMUS M.J. Specific detection and typing of adenovirus types 40 and 41 in stool specimens by dot-blot hybridization. Journal of Clinical Microbiology. 1985;22:934–939. doi: 10.1128/jcm.22.6.934-939.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBY A., WALKER-SMITH J.A., WOOD C.B.S. Studies on the immunoglobulin containing cells and intraepithelial lymphocytes in the small intestinal mucosa of infants with the post-gastroenteritis syndrome. Australian Paediatric Journal. 1976;12:241. [Google Scholar]
- KINGSTON M.E. Biochemical disturbances in breast fed infants with gastroenteritis and dehydration. Journal of Pediatrics. 1973;82:1073. doi: 10.1016/s0022-3476(73)80451-2. [DOI] [PubMed] [Google Scholar]
- KIPPERMAN H., EPHROS M., LAMBDIN M. Aeromonas hydrophila: a treatable cause of diarrhoea. Paediatrics. 1984;73:253–254. [PubMed] [Google Scholar]
- KJELLMAN B., RONG E. Oral solutions for gastroenteritis—optional glucose concentration. Archives of Disease in Childhood. 1982;57:313–315. doi: 10.1136/adc.57.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNISLEY C.V., BEDNAZ-PRASHAD A.J., PICKERING L.K. Detection of rotavirus in stool specimens with mono-clonal and polyclonal antibody-based assay systems. Journal of Clinical Microbiology. 1986;23:897–900. doi: 10.1128/jcm.23.5.897-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNUTTON S., LLOYD D.R., CANDY D.C., MCNEISH A.S. In vitro adhesion of enterotoxigenic Escherichia coli to human intestinal epithelial cells from mucosal biopsies. Infection and Immunity. 1984;44:514–518. doi: 10.1128/iai.44.2.514-518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNUTTON S., LLOYD R.D., CANDY D.C., MCNEISH A.S. Ultrastructural study of adhesion of enterotoxigenic Escherichia coli to erythrocytes and human intestinal epithelial cells. Infection and Immunity. 1984;44:519–527. doi: 10.1128/iai.44.2.519-527.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGASAKA R., SAKUMA Y., CHIBA S., AKIHARA M., HORINO K., NAKAO T. Small round virus-like particles associated with acute gastroenteritis in Japanese children. Journal of Medical Virology. 1980;5:151–160. doi: 10.1002/jmv.1890050209. [DOI] [PubMed] [Google Scholar]
- KOHL S. Yersinia Enterocolitica infections in children. Pediatric Clinics of North America. 1979;26:433–443. doi: 10.1016/s0031-3955(16)33715-4. [DOI] [PubMed] [Google Scholar]
- KOOPMAN J.S., TURKISH V.J., MONTO A.S., GOUVEA V., SRIVASTARA S., ISAACSON R.E. Patterns and aetiology of diarrhoea in three clinical settings. American Journal of Epidemiology. 1984;119:114–123. doi: 10.1093/oxfordjournals.aje.a113712. [DOI] [PubMed] [Google Scholar]
- KRETCHMER N. Child health in the developing world. Paediatrics. 1969;43:4. [PubMed] [Google Scholar]
- KRUGER N., KRAUS C., TILLMAN W., SCHROTER W. Gehauftes Auftreten von Yersiniensepsi bei haemosiderose. Monatsschr Kinderheilkd. 1985;133:876–878. [PubMed] [Google Scholar]
- KUNIN A.S., SURAWICZ B., SIMS E.A.H. Decrease in serum potassium concentration and the appearance of arrhythmias during infusion of potassium with glucose in potassium depleted patients. New England Journal of Medicine. 1962;266:288. doi: 10.1056/NEJM196202012660504. [DOI] [PubMed] [Google Scholar]
- KURITSKY J.N., OSTERHOLM M.T., GREENBERG H.B., KORLATH J.A., GODES J.R., HEDBERG C.W., FORFANG J.C., KAPIKIAN Z.A., MCCULLOUGH J.C., WHITE K.E. Norwalk gastroenteritis: a community outbreak associated with bakery product consumption. Annals of Internal Medicine. 1984;100:519–521. doi: 10.7326/0003-4819-100-4-519. [DOI] [PubMed] [Google Scholar]
- KURTZ J.B., LEE T.W. Human astrovirus serotypes [letter] Lancet. 1984;ii:1405. doi: 10.1016/s0140-6736(84)92101-9. [DOI] [PubMed] [Google Scholar]
- KURTZ J.B., LEE T.W., PICKERING D. Astrovirus associated gastroenteritis in children's ward. Journal of Clinical Pathology. 1977;30:948. doi: 10.1136/jcp.30.10.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURTZ J.B., LEE T.W., CRAIG J.W., REED S.E. Astrovirus infection in volunteers. Journal of Medical Virology. 1979;3:221–230. doi: 10.1002/jmv.1890030308. [DOI] [PubMed] [Google Scholar]
- LAMBETH L., MITCHELL J.H. Transmission of human rotavirus gastroenteritis to a monkey. Australian Paediatric Journal. 1975;II:127. [Google Scholar]
- LATTA T. View of rationale and results of treatment of cholera by aqueous and saline injections. Lancet. 1831-1832;ii:274. doi: 10.5546/aap.2013.92. [DOI] [PubMed] [Google Scholar]
- LEE T.W., KURTZ J.B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. Journal of General Virology. 1981;57:421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- LEVINE M.M., BERGQUIST E.G., NALIN D.R., WATERMAN D.H., HORWICK R.B., YOUNG C.R., SOTMAN S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stabile enterotoxins and are noninvasive. Lancet. 1978;i:1119. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- LJUNGH A., WADSTROM T. Aeromonas and Plesiomonas as possible causes of diarrhoea. Infection. 1985;13:169–173. doi: 10.1007/BF01642805. [DOI] [PubMed] [Google Scholar]
- McCLELLAND, D. B. L. and GILMOUR, H., (1976). In Immunology of the Gut. Ciba Foundation Symposium 46 (new series). Elsevier Excerpta Medical North-Holland Amsterdam, p. 362
- MCDOWELL H.P., EVANS JONES G. Is gradual reintroduction of milk feeds necessary in gastroenteritis? Lancet. 1985;i:690. doi: 10.1016/s0140-6736(85)91343-1. [DOI] [PubMed] [Google Scholar]
- MACFADYEAN F., STOCKMAN S. Vol. 3. HMSO; London: 1913. (Report of the Departments Committee Appointed by the Board of Agriculture and Fisheries to Enquire into Epigastric Absorption). [Google Scholar]
- MCLAUGHLAN P., ANDERSON K.J., WIDDOWSON E.M. Effect of heat on the anaphylactic-sensitising capacity of cow's milk, goat's milk, and various infant formulae fed to guinea pigs. Archives of Diseases in Childhood. 1981;56:165–171. doi: 10.1136/adc.56.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNEISH A.S., TURNER P., FLEMING J., EVANS N. Mucosal adherence of human enteropathogenic Escherichia coli. Lancet. 1975;ii:946–948. doi: 10.1016/s0140-6736(75)90360-8. [DOI] [PubMed] [Google Scholar]
- MADELEY C.R., COSGROVE B.P. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;ii:451. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- MADELEY C.R., COSGROVE B.P. Caliciviruses in man. Lancet. 1976;i:199–200. doi: 10.1016/s0140-6736(76)91309-x. [DOI] [PubMed] [Google Scholar]
- MADELEY C.R., COSGROVE B.P., BELL E.J., FALLOW R.J. Stool viruses in babies in Glasgow. Journal of Hygiene. 1975;78:261. doi: 10.1017/s0022172400056151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIR N.S., FOX E. Yersiniosis. Public Health Laboratory Service, HMSO; London: 1981. [Google Scholar]
- MAIR N.S., FOX E. Yersiniosis. Public Health Laboratory Service, HMSO; London: 1986. [Google Scholar]
- MAIYA P.P., PEREIRA S.M., MATHAN M., BHAT P., ALBERT M.J., BAKER S.J. Acute gastroenteritis in infancy and early childhood in Southern India. Archives of Disease in Childhood. 1976;52:482. doi: 10.1136/adc.52.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALUENDA C., PHILLIPS A.D., BRIDDON A., WALKER-SMITH J.A. Quantitative analysis of small intestinal mucosa in cow's milk-sensitive enteropathy. Journal of Pediatric Gastroenterology and Nutrition. 1984;3:349–357. doi: 10.1097/00005176-198406000-00008. [DOI] [PubMed] [Google Scholar]
- MANUEL P.D., WALKER-SMITH J.A. Decline of hypernatraemia as a problem in gastroenteritis. Archives of Disease in Childhood. 1980;55:124–127. doi: 10.1136/adc.55.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANUEL P.D., WALKER-SMITH J.A. A comparison of the three infant feeding formula for the prevention of delayed recovery after infantile gastroenteritis. Acta Paediatrica Belgica. 1981;34:13–20. [PubMed] [Google Scholar]
- MANUEL P.D., LUCAS MUKHTAR D.J., WALKER-SMITH J.A. Transient monosaccharide intolerance in infants with acute and protracted diarrhoea. Journal of Pediatric Gastroenterology and Nutrition. 1984;3:41–46. doi: 10.1097/00005176-198401000-00011. [DOI] [PubMed] [Google Scholar]
- MATA L., SIMHON A., URRUTIA J.J., KRONMAL R.A.J., FERNANDEZ R., GARCIA B. Epidemiology of rotaviruses in cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of 3 years. Journal of Infectious Diseases. 1983;148:452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- MATHAN M., MATHAN V.I., SWAMINATHAN S.P., YESUDOSS S., BAKER S.J. Pleomorphic viruslike particles in human faeces. Lancet. 1975;i:1068–1069. doi: 10.1016/S0140-6736(75)91832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS T.H.J., NAIR C.D.G., LAWRENCE M.K., TYRRELL D.J.A. Antiviral activity in milk of possible clinical importance. Lancet. 1976;ii:1387. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- MAVROMICHALIS J., EVANS N., MCNEISH A.S., BRYDEN A.S., DAVIES H.A., FLEWETT T.H. Intestinal damage in rotavirus and adenovirus gastroenteritis assessed of D-xylose malabsorption. Archives of Disease in Childhood. 1977;52:549. doi: 10.1136/adc.52.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELBY K., SLORDAHL S., GUTTERBERG T.J., NORDBO S.A. Septicaemia due to Yersinia enterocolitica after oral overdoses of iron. British Medical Journal. 1982;285:467–468. doi: 10.1136/bmj.285.6340.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLETON P.J., SZYMANSKI M.T., ABBOTT G.D. Orbivirus acute gastroenteritis in infancy. Lancet. 1974;i:1241. [Google Scholar]
- MIDDLETON P.J., PETRIC M., HEWITT C.M. Counterimmunoelectro-osmophoresis for the detection of infantile gastroenteritis virus (orbi-group) antigen and antibody. Journal of Clinical Pathology. 1976;29:191. doi: 10.1136/jcp.29.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIOTTI P.G., EIDEN J., YOLKEN R.H. Comparative efficiency of commercial immunoassays for the diagnosis of Rotavirus gastroenteritis during the course of infection. Journal of Clinical Microbiology. 1985;22:693–698. doi: 10.1128/jcm.22.5.693-698.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITTAL S.K. Diarrhoea and Malnutrition. Butterworths; London: 1986. How protective is breast feeding in diarrhoeal diseases? pp. 214–221. Chapter 22. [Google Scholar]
- MOENGINAH P.A., SOENARTO J., BACHTIN M., SUTRISNO D., SUTARYO, ROHDE J.E. Oral sucrase therapy for diarrhoea. Lancet. 1975;ii:323. doi: 10.1016/s0140-6736(75)92756-7. [DOI] [PubMed] [Google Scholar]
- MOFFETT H.L., SHULENBERGER H.K., BURKHOLDER E.R. Epidemiology and aetiology of severe infantile diarrhoea. Journal of Pediatrics. 1968;72:I. doi: 10.1016/s0022-3476(68)80394-4. [DOI] [PubMed] [Google Scholar]
- MOLDE P., ZISSIS G., BUTZLER J.-P., MUTWEWINGABO A., ANDRE F.E. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;ii:108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- MOORE E.E.M. Shigella sonnei septicaemia in a neonate. British Medical Journal. 1974;i:22. doi: 10.1136/bmj.1.5896.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORENS D.M., ZWEIGHAFT R.M., VERNN T.M., GARY G.W., ESLIEN J.J., WOOD B.T., HOLMAN R.C., DOLIN R. A waterbourne outbreak of gastroenteritis with secondary person-to-person spread. Association with a viral agent. Lancet. 1979;i:964–966. doi: 10.1016/s0140-6736(79)91734-3. [DOI] [PubMed] [Google Scholar]
- MORSE D.L., GUZEWICH J.J., HANRAHAN J.P., STRICOF R., SHAYEGANI M., DEIBEL R., GRABAU J.C., NOWAK N.A., HERRMAN J.E., CUKOR G., BLACKLOW N.R. Widespread outbreaks of clam and oyster-associated gastroenteritis: role of Norwalk virus. New England Journal of Medicine. 1986;314:678–681. doi: 10.1056/NEJM198603133141103. [DOI] [PubMed] [Google Scholar]
- MURPHY A.M., ALBERY M., CREWE E. Rotavirus infections of neonates. Lancet. 1977;ii:1149. doi: 10.1016/S0140-6736(77)91538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAZER H., RICE S., WALKER-SMITH J.A. Clinical associations of astro virus in childhood. Journal of Pediatric Gastroenterology and Nutrition. 1983;1:555–558. doi: 10.1097/00005176-198212000-00018. [DOI] [PubMed] [Google Scholar]
- NAZER H., PRICE E.H., HUNT G.H., WALKER-SMITH J.A. Clinical association of Aeromonas spp. in fecal specimens from children. Clinical Paediatrics. 1986;25:516–519. doi: 10.1177/000992288602501006. [DOI] [PubMed] [Google Scholar]
- NEILANDS J.B. Microbial iron compounds. Annual Reviews of Biochemistry. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- NELSON J.D., HALTALIN R.C. Accuracy of diagnosis of bacterial diarrhoeal disease by clinical features. Journal of Pediatrics. 1971;78:519. doi: 10.1016/s0022-3476(71)80240-8. [DOI] [PubMed] [Google Scholar]
- OATES R.K. Infant feeding practices. British Medical Journal. 1973;ii:762. doi: 10.1136/bmj.2.5869.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV F., ORSKOV I., EVANS D.J., SACK B.J., SACK D.A., WADSTROM T. Special Escherichia coli serotypes among enterotoxigenic Med. Microbiology and Immunology. 1976;162:73–80. doi: 10.1007/BF02121318. [DOI] [PubMed] [Google Scholar]
- PAI C.H., SHAHRABADI M.S., INCE B. Rapid diagnosis of rotavirus gastroenteritis by a commercial latex agglutination test. Journal of Clinical Microbiology. 1985;22:846–850. doi: 10.1128/jcm.22.5.846-850.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVER W.K., CAUL E.O., ASHLEY C.R., CLARKE S.K.R. A small virus in human faeces. Lancet. 1973;i:237. doi: 10.1016/s0140-6736(73)90072-x. [DOI] [PubMed] [Google Scholar]
- PERRY R.D., BRUBAKER R.R. Accumulation of iron by yersinia. Journal of Bacteriology. 1979;137:1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS, A. D. (1982). In Basic Science in Gastroenterology, Glaxo, 65–87
- PHILLIPS A.D., WALKER-SMITH J.A. Delayed recovery in childhood. In: WALKER-SMITH J.A., MCNEISH A.S., editors. Diarrhoea and Malnutrition in Childhood. Butterworths; 1986. pp. 107–112. [Google Scholar]
- PHILLIPS A.D., RICE S.J., FRANCE N.E., WALKER-SMITH J.A. Bacteria on duodenal lymph follicle from child with diarrhoea. Lancet. 1978;i:454. doi: 10.1016/s0140-6736(78)91256-4. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A.D., AVIGAD S., RICE S.J., FRANCE N.E., WALKER-SMITH J.A. Microvillous Surface area in secondary disaccharidase deficiency. Gut. 1980;21:44. doi: 10.1136/gut.21.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A.D., RICE S., WALKER-SMITH J.A. Astrovirus with human small mucosa. Gut. 1982;23:10. [Google Scholar]
- PLACZEK M., WALKER-SMITH J.A. Comparison of two feeding regimes following acute gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition. 1984;3:245–248. doi: 10.1097/00005176-198403000-00014. [DOI] [PubMed] [Google Scholar]
- PORTNOY B.L., DUPONT H.L., PRUITT D., ABDO J.A., Rodriquez J.T. Drugs and diarrhoea. Journal of the American Medical Association. 1976;16:844. [Google Scholar]
- RAHAMAN M.M., AZIZ K.M.S., PATWARI K. Diarrhoeal mortality in two Bangladeshi villages with and without community-based oral rehydration therapy. Lancet. 1979;ii:809. doi: 10.1016/s0140-6736(79)92172-x. [DOI] [PubMed] [Google Scholar]
- RAHILLY P.M., SHEPHERD R., CHALLIS D., WALKER-SMITH J.A., MANLY J. Clinical comparison between glucose and sucrose additions to a basic electrolyte mixture in the outpatient management of acute gastroenteritis in children. Archives of Disease in Childhood. 1976;51:152. doi: 10.1136/adc.51.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUCHER H.S., EICHENFIELD A.H., HODES H.L. Treatment of salmonella gastroenteritis in infants. The significance of bacteria. Clinical Pediatrics. 1983;22:601–604. doi: 10.1177/000992288302200901. [DOI] [PubMed] [Google Scholar]
- REIMAN H.A., HODGES J.H., PRICE A.H. Epidemic diarrhoea, nausea and vomiting of unknown cause. Journal of American Medical Association. 1945;127:1. [Google Scholar]
- REES L., BROOK C.G.D. Gradual re-introduction of full strength milk after acute gastroenteritis in children. Lancet. 1979;ii:770–771. doi: 10.1016/s0140-6736(79)91220-0. [DOI] [PubMed] [Google Scholar]
- RICHMOND S.J., CAUL E.O., DUNN S.M., AGHLEY C.R., CLARKE S.K.R. An outbreak of gastroenteritis in young children caused by adenoviruses. Lancet. 1979;i:1178–1180. doi: 10.1016/s0140-6736(79)91853-1. [DOI] [PubMed] [Google Scholar]
- ROBINS-BROWNE R.M., PRPIC J.K. Effect of iron and Desferrioxamine on infections with Yersinia enterocolitica. Infection and Immunity. 1985;47:774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J.E., HARVEY B.A., SOOTHILL J.F. Phagocytosis and killing of bacteria and yeast by human milk cells after opsonization in aqueous phase of milk. British Medical Journal. 1978;i:1443. doi: 10.1136/bmj.1.6125.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIQUEZ W.J., KIM H.W., ARROBIO J.O., BRANDT C.D., CHANOCK R.M., KAPIKIAN A.Z., WYATT R.G., PARROTT R.H. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. Journal of Pediatrics. 1977;91:188. doi: 10.1016/S0022-3476(77)80810-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHDE, J.E., and NORTHRUP, R. S., (1976). Taking Science Where the Diarrhoea is, Ciba Foundation Symposium 42 (new series). Acute Diarrhoea in Childhood. Elsevier Excerpta Medica North-West Holland, 358 [DOI] [PubMed]
- ROWLAND M.G.M., BARRELL R.A.E., WHITEHEAD R.G. Bacterial contamination in traditional Gambian weaning foods. Lancet. 1978;i:136–138. doi: 10.1016/s0140-6736(78)90432-4. [DOI] [PubMed] [Google Scholar]
- RUTTER J.M., BURROWS M.R., SELLWOOD R., GIBBONS R.A. A genetic basis for resistance to enteric disease caused by E. coli. Nature (Lond.) 1975;257:135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- RYDER R.W., SACK D.A., KAPIKIAN A.Z., MCLAUGHLIN J.C., CHAKRABORTY J., RAHMAN A.S. Enterotoxigenic Escherichia coli and reovirus-like agent in rural Bangladesh. Lancet. 1976;i:659. doi: 10.1016/s0140-6736(76)92776-8. [DOI] [PubMed] [Google Scholar]
- SANTOSHAM M., SACK R.B., LOCHLEAR E., FOSTER S., GARRET S., ROUSEY D., EVANS S.S., BLACK R. Storing oral rehydration solution. Lancet. 1982;i:797. doi: 10.1016/s0140-6736(82)91836-0. [DOI] [PubMed] [Google Scholar]
- SCHLOSSMAN A. Ueber die giftwirkung des artfremden eiweisses in der milch auf den organismus des sauglings. Arch Kinderheilkd. 1905;42:99–103. [Google Scholar]
- SCHREIBER D.S., BLACKLOW N.R., TRIER J.S. Lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. New England Journal of Medicine. 1975;288:1318. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- SCHREIBER D.S., TRIER J.S., BLACKLOW N.R. Recent advances in viral gastroenteritis. Gastroenterology. 1977;73:174. doi: 10.1016/S0016-5085(19)32293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLWOOD R., GIBBONS R.A., JONES G.W., RUTTER J.M. Adhesion of enteropathogenic Escherichia E coli to pig intestinal brush borders; the existence of two pig phenotypes. Journal of Medical Microbiology. 1975;8:405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E.O. Yersinia enterocolitica septicemia in normal infants. American Journal of Diseases in Children. 1981;135:477–478. doi: 10.1001/archpedi.1981.02130290073025. [DOI] [PubMed] [Google Scholar]
- SHAW R.D., ESTES M.K., STONER-MA D., GREENBERG H.B. Specific enzyme linked immunoassay for rotavirus serotypes 1 and 3. Journal of Clinical Microbiology. 1985;22:286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEARS P., HART C.A., COULTER J.B.S., BROADHEAD A. A High Prevalence of Antibiotic Resistance in Commensal Bacteria of Children in Sudan—A Cause for Concern. British Paediatric Association; York: 1987. [Google Scholar]
- SHEEKY T.W., ARTENSTEIN M.S., GREEN R.W. Small intestinal mucosa in certain viral diseases. Journal of the American Medical Association. 1964;190:1023. doi: 10.1001/jama.1964.03070250005001. [DOI] [PubMed] [Google Scholar]
- SHEPHERD R.W., TRUSLOW S., WALKER-SMITH J.A., BIRD R., CUTTING W., DARNELL R., BARKER C.M. Infantile gastroenteritis: a clinical study of reovirus-like agent infection. Lancet. 1975;ii:1082. doi: 10.1016/s0140-6736(75)90446-8. [DOI] [PubMed] [Google Scholar]
- SHIGA K. Ueber den Dysenteriebacillus (bacillus dysenteriae) Zentralblatt fur Bacteriologie. 1898;24:913. [Google Scholar]
- SHINER M., BALLAR J., SMITH M.E. The small intestinal mucosa in cow's milk allergy. Lancet. 1975;i:136. doi: 10.1016/s0140-6736(75)91431-2. [DOI] [PubMed] [Google Scholar]
- SIMHON A., MATA L. Fecal rotavirus, adenoviruses, coronavirus-like particles and small round viruses in a cohort of rural Costa Rican children. American Journal of Tropical Medicine and Hygiene. 1985;34:931–936. doi: 10.4269/ajtmh.1985.34.931. [DOI] [PubMed] [Google Scholar]
- SIMHON A., CHRYSTIE I.L., TOTTERDELL B.M., BANATVALA J.E., RICE S.J., WALKER-SMITH J.A. Sequential Rotavirus diarrhoea caused by virus of same subgroup. Lancet. 1981;ii:1174. doi: 10.1016/s0140-6736(81)90627-9. [DOI] [PubMed] [Google Scholar]
- SIMHON A., MATA L., VIVES M., RIVERA L., VARGAS S., RAMIREZ G., LIZANO L., CATARINELLA G., AZOFEIFA J. Low endemicity and low pathologenicity of rotaviruses among rural children. Costa Rica. 1985;152:1134–1141. doi: 10.1093/infdis/152.6.1134. [DOI] [PubMed] [Google Scholar]
- SINHA A.K., TYRELL D.A. Changes in the clinical pattern of gastrointestinal infection in North West London. British Journal of Clinical Practice. 1973;27:45. [PubMed] [Google Scholar]
- SIVAKUMAR B., REDDY V. Absorption of labelled Vitamin A in children during infection. British Journal of Nutrition. 1972;27:299. doi: 10.1079/bjn19720094. [DOI] [PubMed] [Google Scholar]
- SKIRROW M.B. Campylobacter enteritis: a ‘new’ disease. British Medical Journal. 1977;ii:9. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNODGRASS D.R., GRAY E.W. Detection and transmission of 30 nm virus particles (astroviruses) in the faeces of lambs with diarrhoea. Archives of Virology. 1977;55:287–291. doi: 10.1007/BF01315050. [DOI] [PubMed] [Google Scholar]
- SNODGRASS D.R., ANGUS K.W., GRAY E.W., MENZIES J.D., PAUL J. Pathogenesis of diarrhoea caused by astrovirus infections in lambs. Archives of Virology. 1979;60:217–226. doi: 10.1007/BF01317493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J.D., MERSON M.H. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bulletin of the World Health Organisation. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- SOENARTO Y., SEBODO T., SURYANTORO P., KRISNOMURTI, HAKSOHUSODO S., ILYAS KUSNIYO RISTANTO, ROMAS M.A., NOERHAJATI, MUSWIROH S., ROHDE J.E., RYAN N.J., LUKE R.K.J., BARNES G.L., BISHOP R.F. Bacteria, parisitiv agents and rotaviruses associated with acute diarrhoea in hospital in-patient Indonesian children. Transcriptions of the Royal Society for Tropical Medicine and Hygiene. 1983;77:724–730. doi: 10.1016/0035-9203(83)90215-8. [DOI] [PubMed] [Google Scholar]
- SOOTHILL J.F. Breast-feeding: the immunological argument. British Medical Journal. 1976;i:1466. doi: 10.1136/bmj.1.6023.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCE L., FANVEL M., BOUCHARD S. Test for reovirus-like agent. Lancet. 1975;ii:322. doi: 10.1016/s0140-6736(75)92752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER I.W.F., COSTER M.E.E. The epidemiology of gastroenteritis in infancy. South African Medical Journal. 1969;2:1391. [PubMed] [Google Scholar]
- STOLIAR O.A., PELLEY R.P., KANIECKI-GREEN E., KLAUR M.H., CARPENTER C.C.J. Secretory IgA against enterotoxins in breast-milk. Lancet. 1976;i:1258. doi: 10.1016/s0140-6736(76)91735-9. [DOI] [PubMed] [Google Scholar]
- STORR J., RICE S., PHILLIPS A.D., PRICE E., WALKER-SMITH J.A. Clinical associations of Norwalk like virus in the stools of children. Journal of Pediatric Gastroenterology and Nutrition. 1986;5:576–581. doi: 10.1097/00005176-198607000-00012. [DOI] [PubMed] [Google Scholar]
- SUNDERLAND R., EMERY J.L. Apparent disappearance of hypernatraemic dehydration from infant deaths in Sheffield. British Medical Journal. 1979;ii:575–576. doi: 10.1136/bmj.2.6190.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNSHINE P., KRETCHMER N. Studies of small intestine during development. Pediatrics. 1964;34:38. [PubMed] [Google Scholar]
- SUPRAPTI R., NAFSIAH T., RUMINI M. Microbiological parasitic and epidemiological considerations on infantile diarrhoeal disease. Paediatrica Indonesiana. 1968;8:133. [Google Scholar]
- SUZUKI H., KONNO T. Reovirus-like particles in jejunal mucosa of a Japanese infant with acute infectious nonbacterial gastroenteritis. Tohoku Journal of Experimental Medicine. 1975;115:199. doi: 10.1620/tjem.115.199. [DOI] [PubMed] [Google Scholar]
- TALLETT S., MACKENZIE C., MIDDLETON P., KERZNER B., HAMILTON R. Clinical, laboratory and epidemiologic features of a viral gastroenteritis in infants and children. Paediatrics. 1977;60:217. [PubMed] [Google Scholar]
- TAO H., GUANGMU C., CHANGAN W., HENLI Y., ZHAOYING F., TUNGXIN C., ZINYI C., WEIWE Y., XUEJAN C., SHUASEN D., XIAOQUANG L., WEICHENG C. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984;i:1139–1142. [PubMed] [Google Scholar]
- TAUWERS S., DE BOECK M., BUTZLER J.P. Campylobacter enteritis in Brussells. Lancet. 1978;i:604. doi: 10.1016/s0140-6736(78)91045-0. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.B., ECHEVARRIA P., BLASER M.N. Polymicrobial aetiology of travellers’ diarrhoea. Lancet. 1985;i:381–383. doi: 10.1016/s0140-6736(85)91397-2. [DOI] [PubMed] [Google Scholar]
- TORRES-PINEDO R.L., LAVASTIDA M., RIVERA C.L. Studies on infant diarrhoea. A comparison of the effects of milk feeding and intravenous therapy upon the composition and values of the stool and urine. Journal of Clinical Investigation. 1966;45:469. doi: 10.1172/JCI105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREANOR, J.J., MADORE, H. P. and DOLIN, R., (1986). Development of a monoclonal antibody to the Snow Mountain agent of gastroenteritis (abstract 109). In Program and Abstracts of the 26th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D C, American Society for Microbiology
- TRIPP J.H., WILMERS M.J., WHARTON B.A. Gastroenteritis: a continuing problem of child health in Britain. Lancet. 1977;ii:233. doi: 10.1016/s0140-6736(77)92846-x. [DOI] [PubMed] [Google Scholar]
- TROUNCE J.Q., WALKER-SMITH J.A. Sugar intolerance complication acute gastroenteritis. Archives of Disease in Childhood. 1985;60:986–990. doi: 10.1136/adc.60.10.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROUNCE J.Q., WALKER-SMITH J.A. Dextrolyte in management of children with acute gastroenteritis. The Practitioner. 1985;229:80–82. [PubMed] [Google Scholar]
- VANTRAPPEN G., AGG H.O., PONETTE E., GEBOES K., BERTRAND P.H. Yersinia enteritis and enterocolitis: gastroenterological aspect. Gastroenterology. 1977;72:220. [PubMed] [Google Scholar]
- VESIKARI T., ISOLAURI E., DELEM A., D'HONDT E., ANDRE F.E., ZISSIS G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983;ii:807–881. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]
- VESIKARI T., ISOLAURE E., D'HONDT E., DELEM A., ANDRE F.E., ZISSIS G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;i:977–980. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- VESIKARI T., ISOLAURI E., DELEM A. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. Journal of Pediatrics. 1985;107:189–195. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- VON BONSDORFF C.H., HOVI T., MAKELA P., HOVI L., TEVALVOTO-AARNIO M. Rotavirus associated with acute gastroenteritis in adults. Lancet. 1976;ii:432. doi: 10.1016/s0140-6736(76)92445-4. [DOI] [PubMed] [Google Scholar]
- VON GRAEVENITZ A., MENSCH A.H. The genus Aeromonas in human bacteriology; report of 30 cases and review of the literature. New England Journal of Medicine. 1986;278:245–249. doi: 10.1056/NEJM196802012780504. [DOI] [PubMed] [Google Scholar]
- VONDERFECHT S.L., MISKUFF R.L., EIDEN J.E., YOLKEN R.H. Enzyme immunoassay inhibition assay for the detection of rat rotavirus-like agent in intestinal and fecal specimens obtained from diarrheic rats and humans. Journal of Clinical Microbiology. 1985;22:726–730. doi: 10.1128/jcm.22.5.726-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER, A. C. (1971). The aboriginal child: gastroenteritis. Presented at The Ross Conference of Aboriginal Child, Sydney
- WALKER-SMITH J.A. Transient gluten intolerance. Archives of Diseases of Childhood. 1970;45:523. doi: 10.1136/adc.45.242.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER-SMITH J.A. Gastroenteritis. Medical Journal of Australia. 1972;i:329. doi: 10.5694/j.1326-5377.1972.tb46805.x. [DOI] [PubMed] [Google Scholar]
- WALKER-SMITH J.A. Cow's milk protein intolerance; Transient food intolerance of infancy. Archives of Diseases of Childhood. 1975;50:347. doi: 10.1136/adc.50.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER-SMITH J.A., KAMATH K.R., STOBO E.A. Some observations on monosaccharide malabsorption. Australian Paediatric Journal. 1972;8:157. [Google Scholar]
- WALKER-SMITH J.A., HARRISON M., KILBY A., PHILLIPS A., FRANCE N.E. Cow's milk sensitive enteropathy. Archives of Diseases in Childhood. 1978;53:375. doi: 10.1136/adc.53.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL M.H. A unified guide to parenteral fluid therapy. Journal of Pediatrics. 1969;75:I. doi: 10.1016/s0022-3476(69)80095-8. [DOI] [PubMed] [Google Scholar]
- WEIL M.H. Editorial Comment. Journal of Pediatrics. 1973;82:1979. [Google Scholar]
- WEINDLING A.M., WALKER-SMITH J.A., BIRD R. Micro-organisms in outpatient infantile gastroenteritis. Archives of Disease in Childhood. 1980;55:185. doi: 10.1136/adc.55.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHARTON, B. A. (1968). Difficulties in The Initial Treatment of Kwashiorkor. Calorie Deficiencies and Protein Deficiencies. Proceedings of a colloquium, Churchill, London, p. 147
- WHEATLEY D. Incidence and treatment of infantile gastroenteritis in general practice. Archives of Disease in Childhood. 1968;43:53. doi: 10.1136/adc.43.227.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELAW A.W., DAVIES H., PARRY J. Electron microscopy fatal adenovirus gastroenteritis. Lancet. 1977;i:361. doi: 10.1016/s0140-6736(77)91158-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS SMITH, H., (1976). Neonatal Escherichia Coli Infections in Domestic Mammals: Transmissibility of Pathogenic Characteristics Acute Diarrhoea in Childhood. Ciba Foundation Symposium 42, p. 45 [DOI] [PubMed]
- WILLIAMS P.H., SEDGEWICK M.I., EVANS N., TURNER P.J., GEORGE R.H., MCNEISH A.S. Adherence of enteropathogenic strain of Escherichia coli to human intestinal mucosa is mediated by colcigenic conjugative plasmid. Infective Immunology. 1978;22:393–402. doi: 10.1128/iai.22.2.393-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION. (1974). World Health Statistics Report, vol. 27, WHO, Geneva, p. 563
- YAP K.L., SABIL D., MUTHU P.A. Huan rotavirus infection in Malaysia: I. a hospital-based study of rotavirus in children with acute gastroenteritis. Journal of Tropical Pediatrics. 1984;30:131–135. doi: 10.1093/tropej/30.3.131. [DOI] [PubMed] [Google Scholar]
- ZAHORSKY J. Hyperemesis Hiemis, or the winter vomiting disease. Archives of Paediatrics. 1929;64:391. [Google Scholar]
- ZOPPI G., DE GANELLO A., GABURRO D. Persistent post-enteritis diarrhoea. European Journal of Paediatrics. 1977;126:225. doi: 10.1007/BF00477048. [DOI] [PubMed] [Google Scholar]
- ZOPPI G., ZAMBONI G., BASSANI N., VAZZOLER G. Gammaglobulin level and soy-protein intake in early infancy. European Journal of Pediatrics. 1979;131:61. doi: 10.1007/BF00442786. [DOI] [PubMed] [Google Scholar]
- ZOYSA DE I., FEACHEM R.G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunisation. WHO Bulletin. 1985;63:569–583. [PMC free article] [PubMed] [Google Scholar]



